Water Hardness Limescale deposits in hot water pipes



















- Slides: 31

Water Hardness

Limescale deposits in hot water pipes and on filament in kettles Ca. CO 3

Water Hardness � Hard water is water that will not easily form a lather with soap. � Hardness is caused by the presence of Ca 2+ or Mg 2+ ions dissolved in the water. These ions react with the soap to form a scum rather than a lather. � The concentration of the Ca 2+ ions is greater than the concentration of any other metal ion in our water. � Water hardness is usually expressed in ppm Ca. CO 3
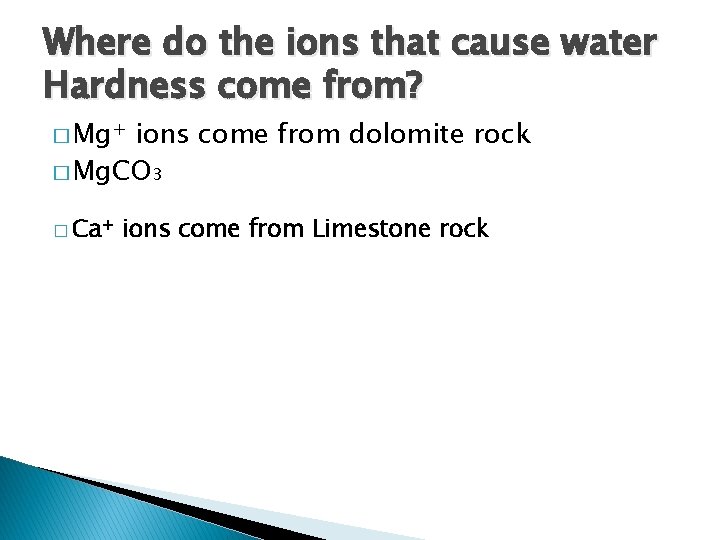
Where do the ions that cause water Hardness come from? � Mg+ ions come from dolomite rock � Mg. CO 3 � Ca+ ions come from Limestone rock

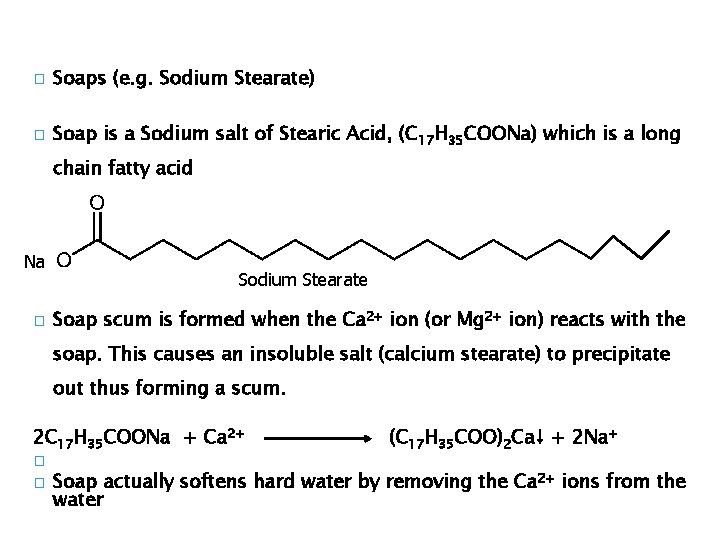
� Soaps (e. g. Sodium Stearate) � Soap is a Sodium salt of Stearic Acid, (C 17 H 35 COONa) which is a long chain fatty acid Na � Sodium Stearate Soap scum is formed when the Ca 2+ ion (or Mg 2+ ion) reacts with the soap. This causes an insoluble salt (calcium stearate) to precipitate out thus forming a scum. 2 C 17 H 35 COONa + Ca 2+ � � (C 17 H 35 COO)2 Ca↓ + 2 Na+ Soap actually softens hard water by removing the Ca 2+ ions from the water

Modern detergents � Made from crude oil � Not affected by hard water ions � Too severe on skin

Soft water � Is water that lathers easily � No Ca 2+ or Mg 2+ ions

Types of Hardness � Temporary Hardness � Permanent Hardness

Temporary Hardness is hardness that can be removed by boiling. Temporary hardness is caused by: ◦ Calcium Hydrogencarbonate Ca(HCO 3)2 ◦ Magnesium Hydrogencarbonate Mg(HCO 3)2

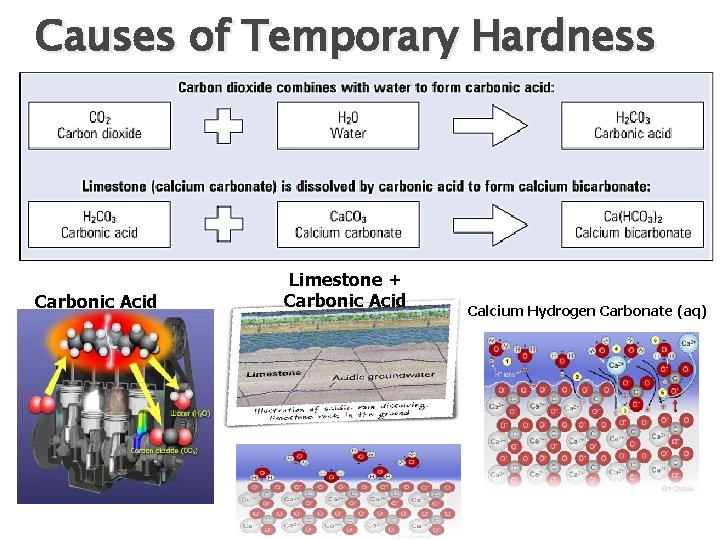
Causes of Temporary Hardness � Temporary � Rainwater � Acidic Hardness can be removed by Boiling slightly acidic due to CO 2 (carbonic acid) rainfall will dissolve limestone (Ca. CO 3) � Forming soluble Calcium Hydrogen Carbonate Ca(HCO 3)2 and Mg (HCO 3)2 � If heated a reaction occurs in which the Ca ions form insoluble Ca. CO 3 � Pg 264 book for equations (must know)

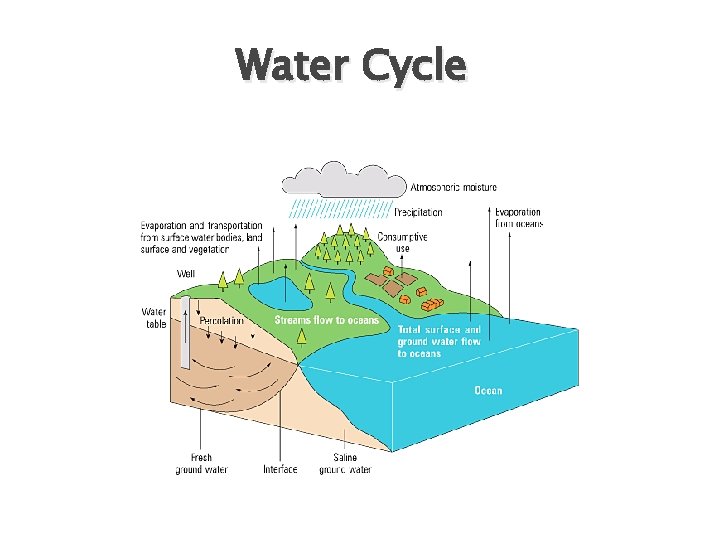
Water Cycle

Causes of Temporary Hardness Carbonic Acid Limestone + Carbonic Acid Calcium Hydrogen Carbonate (aq)

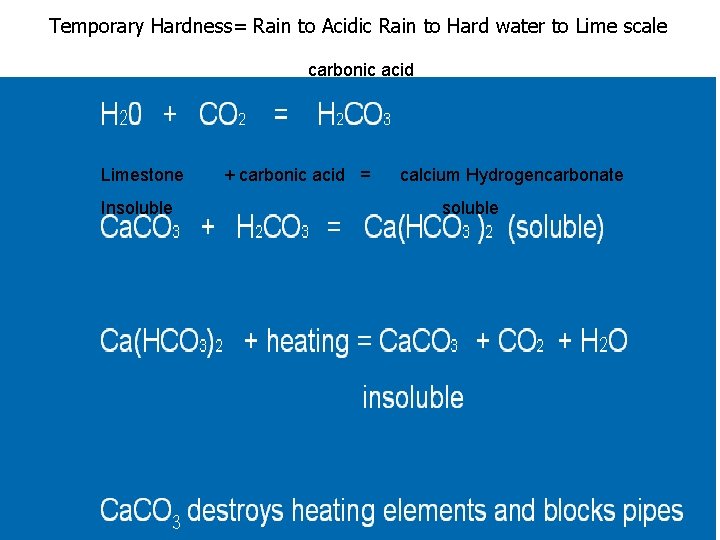
Temporary Hardness= Rain to Acidic Rain to Hard water to Lime scale carbonic acid H 20 + CO 2 = H 2 CO 3 Ca. CO 3 + H 2 CO 3 = Ca(HCO 3 )2 (soluble) Limestone + carbonic acid = calcium Hydrogencarbonate Insoluble Ca(HCO 3)2 + heating = Ca. CO 3 + CO 2 + H 2 O insoluble Ca. CO 3 destroys heating elements and blocks pipes

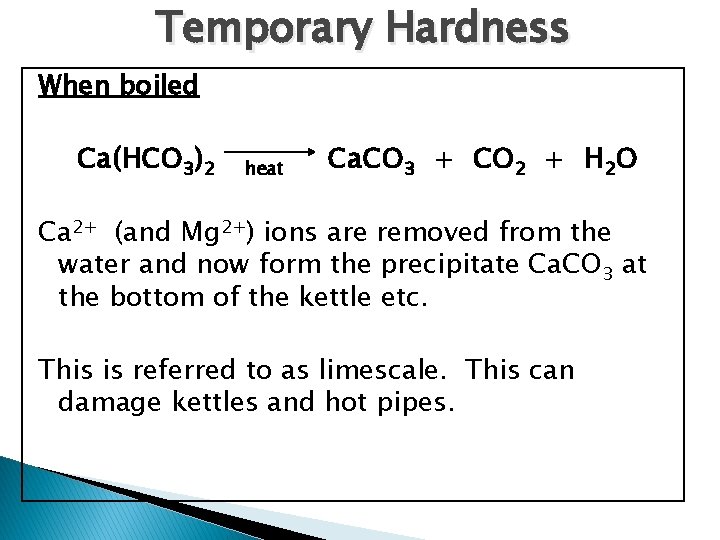
Temporary Hardness When boiled Ca(HCO 3)2 heat Ca. CO 3 + CO 2 + H 2 O Ca 2+ (and Mg 2+) ions are removed from the water and now form the precipitate Ca. CO 3 at the bottom of the kettle etc. This is referred to as limescale. This can damage kettles and hot pipes.

Why Be Concerned About Hard Water? � Hard water does cause soap scum, clogs pipes and clogs boilers as limescale

Permanent hardness � Permanent Hardness is hardness that cannot be removed by boiling. � Permanent hardness is caused by ◦ Calcium Chloride Ca. Cl 2 ◦ Magnesium Chloride Mg. Cl 2 ◦ Calcium Sulfate Ca. SO 4 ◦ Magnesium Chloride Mg SO 4

Hard water disadvantages � Limescale is deposited on heating elements which reduces their efficiency e. g. washing machines and kettles � Limescale blocks pipes � Soap not as efficient � Produces scum

Hard water Advantages � Source of calcium which is good for teeth and bones � Some people believe it tastes nicer � Makes better tasting beer

To soften the hard water it is necessary to remove the calcium and magnesium ions � Addition of washing soda � Distillation � Ion exchanges � Deionisers

Addition of washing soda � Washing soda is hydrated sodium carbonate (Na 2 CO 3. 10 H 2 O) � Calcium ions+ carbonate ions=calcium carbonate (insoluble) � Washing soda is usually included in clothes detergent � Ca 2+ + CO 3 - = Ca. CO (precipitate) 3

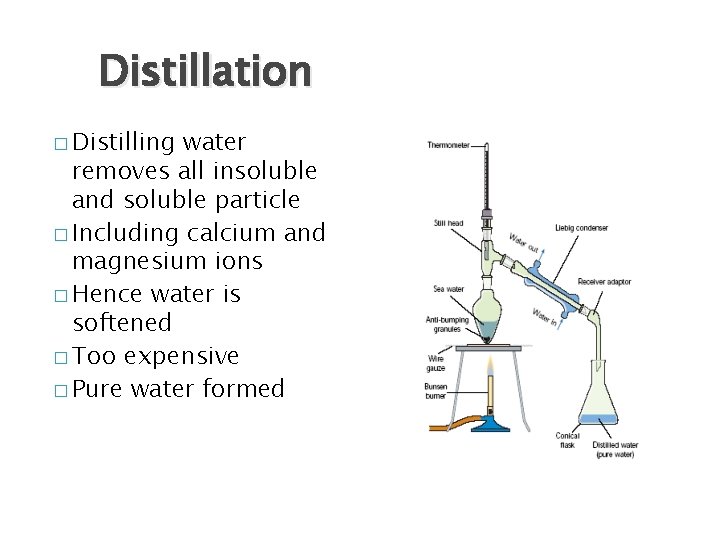
Distillation � Distilling water removes all insoluble and soluble particle � Including calcium and magnesium ions � Hence water is softened � Too expensive � Pure water formed

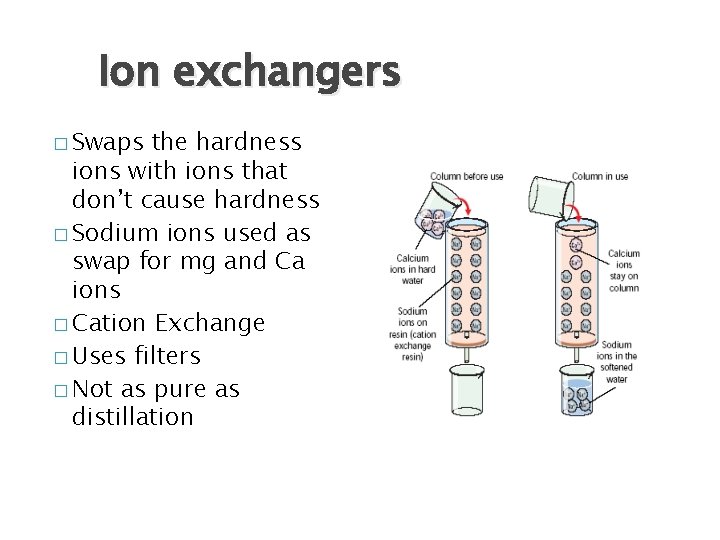
Ion exchangers � Swaps the hardness ions with ions that don’t cause hardness � Sodium ions used as swap for mg and Ca ions � Cation Exchange � Uses filters � Not as pure as distillation


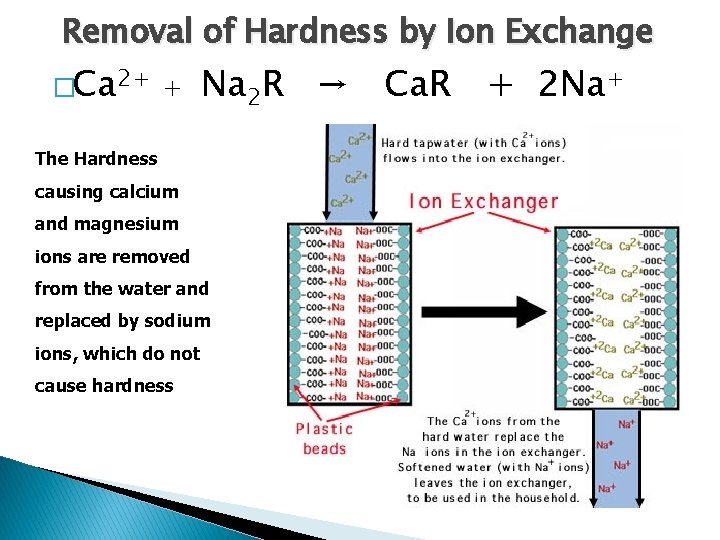
Removal of Hardness by Ion Exchange �Ca 2+ + Na 2 R → Ca. R + 2 Na+ The Hardness causing calcium and magnesium ions are removed from the water and replaced by sodium ions, which do not cause hardness

Deionised water � To make deionised water it must be passed over a mixed bed resin (cation and anion resin) � RH (cation) + Na+ = RNa + H+ � ROH (anion) + CL- = RCL + OH� H+ + OH- = H 2 O � Only ions removed so product not as pure as distillation

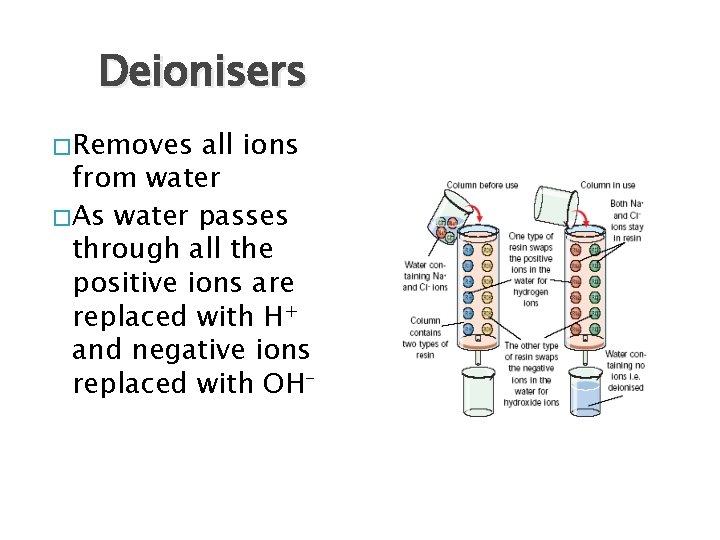
Deionisers � Removes all ions from water � As water passes through all the positive ions are replaced with H+ and negative ions replaced with OH-


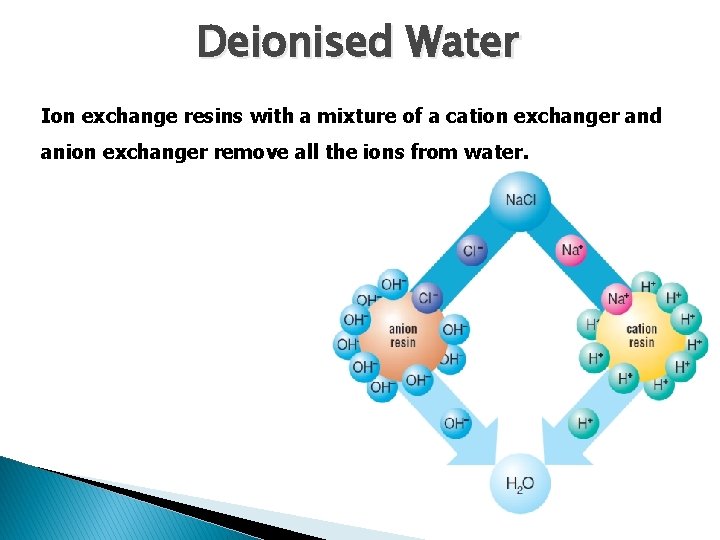
Deionised Water Ion exchange resins with a mixture of a cation exchanger and anion exchanger remove all the ions from water.

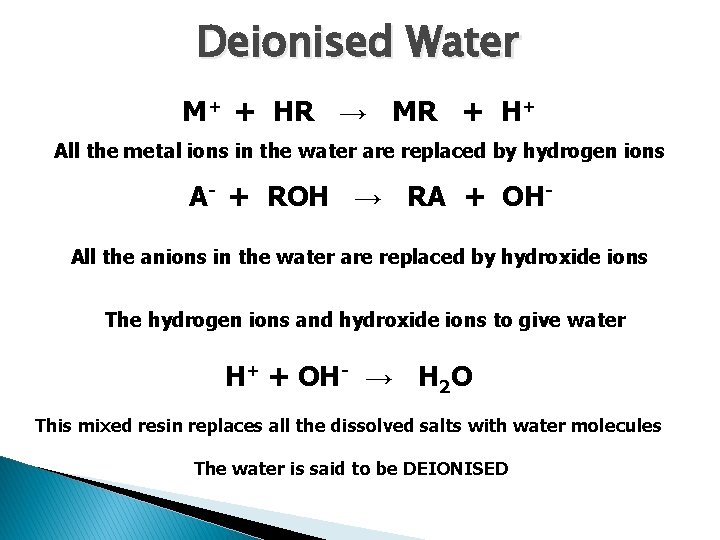
Deionised Water M+ + HR → MR + H+ All the metal ions in the water are replaced by hydrogen ions A- + ROH → RA + OHAll the anions in the water are replaced by hydroxide ions The hydrogen ions and hydroxide ions to give water H+ + OH- → H 2 O This mixed resin replaces all the dissolved salts with water molecules The water is said to be DEIONISED

Distilled vs Deionised � Purer � Dissolved and suspended solids removed � Not as pure � Dissolved gases present � Non-ionic molecules present

Deionised Water and Distilled Water Deionised water is not quite as pure as distilled water Deionised water has all the dissolved ions in the water removed Distilled water has all the dissolved solids and covalent compounds e. g. dissolved gases removed
 Lime soda process of water softening
Lime soda process of water softening How to calculate hardness of water
How to calculate hardness of water How to calculate total hardness
How to calculate total hardness Knockhardy gcse
Knockhardy gcse Hardness of water
Hardness of water What information should a master cleaning schedule contain
What information should a master cleaning schedule contain Removal of water hardness
Removal of water hardness Pseudo hardness of water
Pseudo hardness of water Units of hardness of water
Units of hardness of water Water and water and water water
Water and water and water water White hot vs red hot temperature
White hot vs red hot temperature Principle of hot working process
Principle of hot working process Perbedaan hot lava dan hot lava volcano
Perbedaan hot lava dan hot lava volcano Be either hot or cold
Be either hot or cold Ben has had problems with the pipes in his apartment
Ben has had problems with the pipes in his apartment Types of heat pipes
Types of heat pipes Cpvc pipes in uae
Cpvc pipes in uae Ccdcfe happy birthday
Ccdcfe happy birthday Message queues in rtos
Message queues in rtos During masonry construction the limited access zone must be
During masonry construction the limited access zone must be Pastoral
Pastoral Offshore pipes
Offshore pipes Ence pipes
Ence pipes Lee pipes
Lee pipes Lee pipes
Lee pipes Synerzip
Synerzip Uilleann pipes
Uilleann pipes Open closed tube
Open closed tube Pvc pan flute measurements
Pvc pan flute measurements Flow through pipes
Flow through pipes Minecraft hardness
Minecraft hardness Chapter 11 section 2 stream and river deposits answer key
Chapter 11 section 2 stream and river deposits answer key