Water Chapter 33 What is water Water is













- Slides: 27

Water Chapter 33

What is water? • Water is a compound made up of the elements Hydrogen and Oxygen. • The chemical formula for water is H 2 O. • The test for water is Cobalt Chloride paper. Turns Blue to Pink in the presence of water.

Properties of Water • The freezing point of water is 0 C and the Boiling point is 100 C. • Water expands when it freezes. Ice is less dense than water an it floats. • Water is an excellent solvent. • The test for water is Cobalt Chloride paper. • Water tends to cling to glass

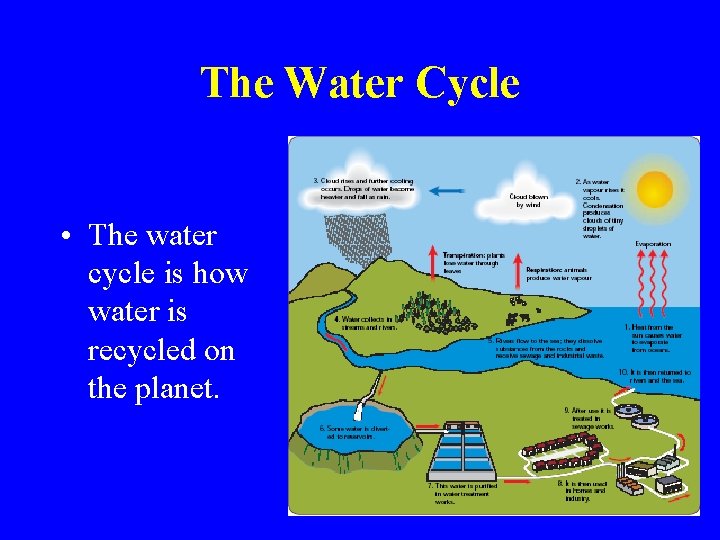
The Water Cycle • The water cycle is how water is recycled on the planet.

Stages of the Water cycle • Heat from the sun causes water to evaporate. • Vapour cools and condenses to form tiny droplets called clouds. • Cooling causes the droplets to join up and fall as rain. • Rainwater soaks through the ground and form rivers and streams. • Rivers carry the water to seas and oceans.

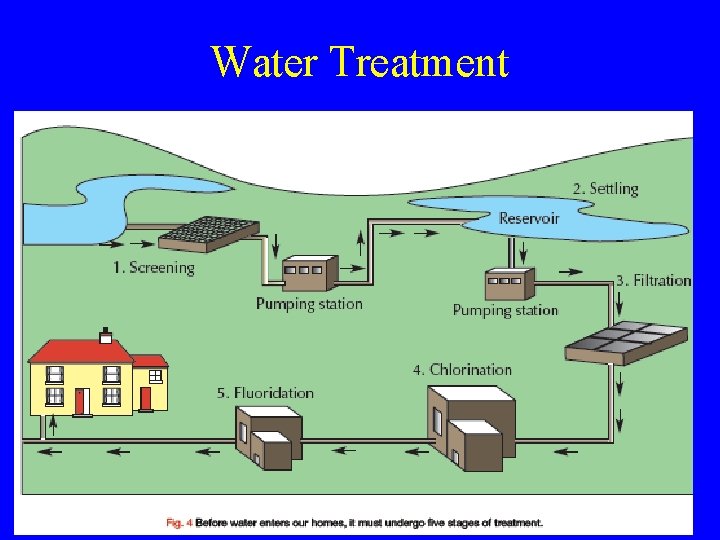
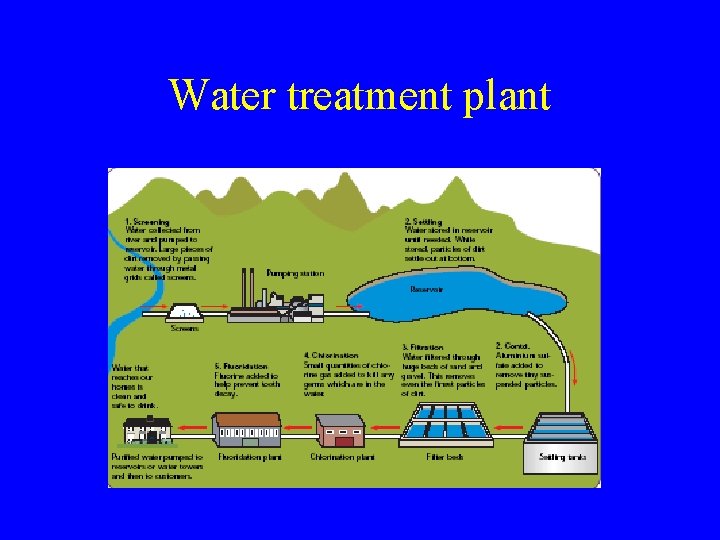
Water Treatment There are 5 stages in the treatment of water 1. 2. 3. 4. 5. Screening-removal of large debris Settling-Water in settling tanks Filtration-water passed through filter beds Chlorination-chlorine kills bacteria Fluoridation-fluorine prevents tooth decay

Water Treatment

Water treatment plant

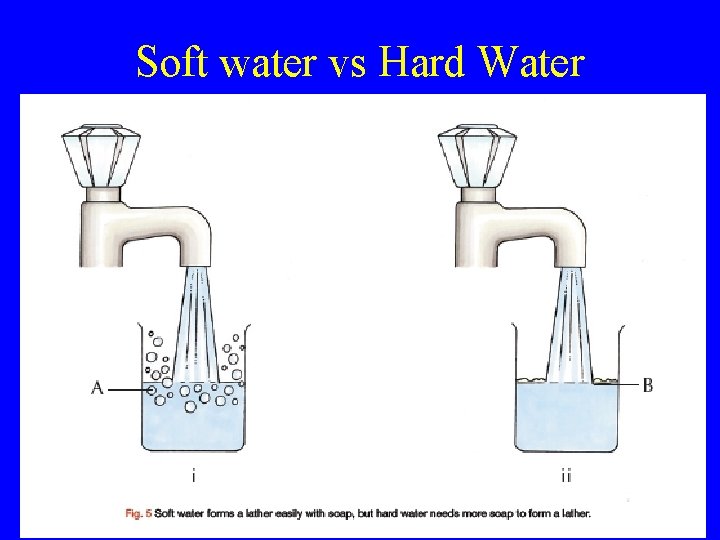
Hard and Soft Water Hard water is water which does not form a lather easily with soap. Hardness in water is caused by the presence of calcium or magnesium ions dissolved in the water. Calcium ions + Soap Scum

Soft water vs Hard Water

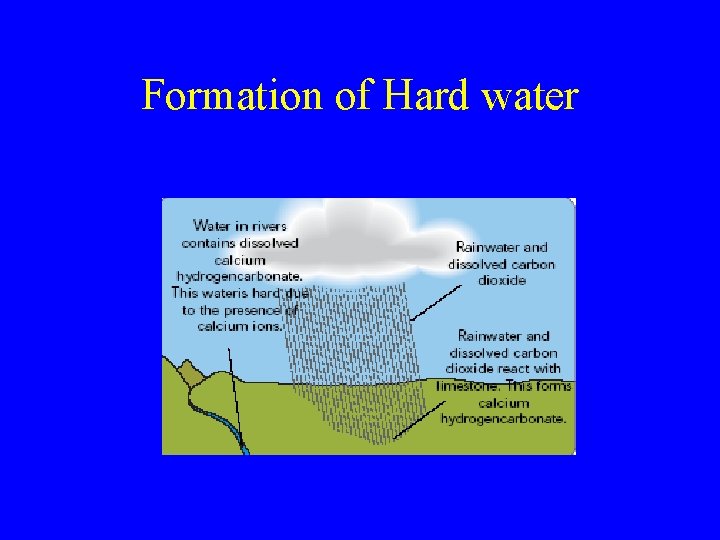
Formation of hard water Hard water is found in limestone areas(calcium Carbonate). The calcium carbonate rock reacts with slightly acidic rainwater to release calcium ions into the water. Calcium Carbonate + rainwater (insoluble) (acidic) Calcium ions in solution (soluble)

Formation of Hard water

Advantages of Hard Water • It provides Calcium for healthy bones and teeth. • It is good for brewing and tanning. • Nicer taste

Disadvantages of Hard water • It blocks pipes and leaves a scale on kettles and boilers. • It wastes soap • It produces scum with soap.


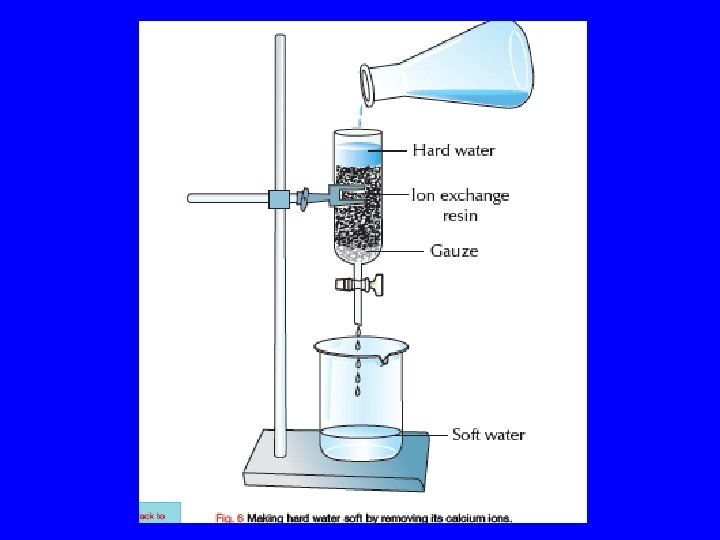
Removal of hardness • Hardness can be removed by passing water through an ion exchange resin or by distillation. Positive ions are replaced by H+ ions. Negative ions are replaced by OH- ions. H+ + OH- H 2 O

Making Hard Water Soft by the removal of Calcium ions


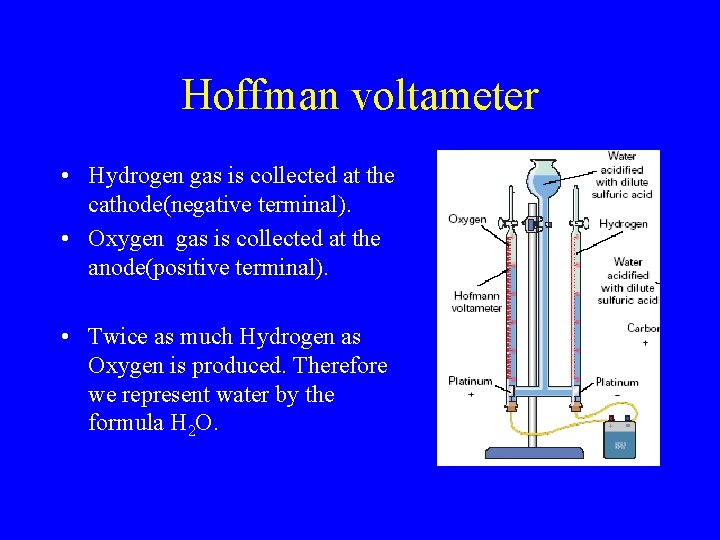
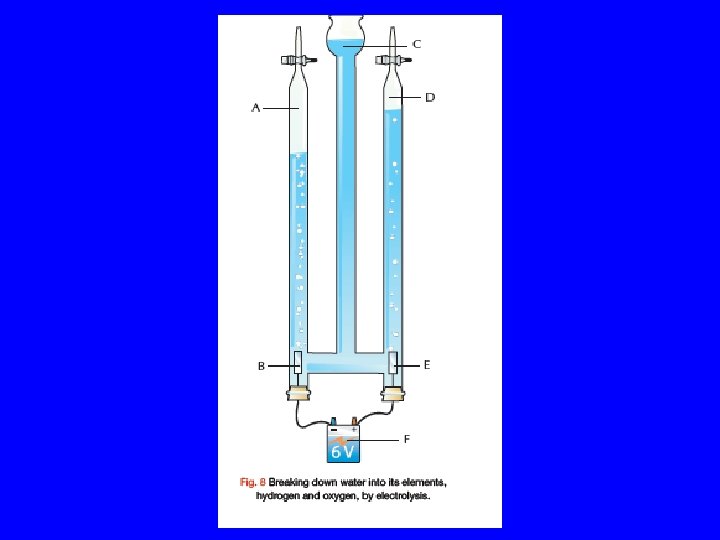
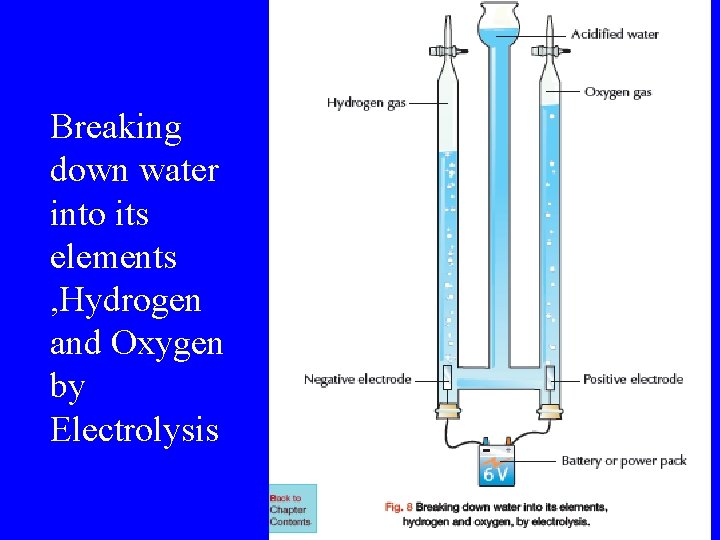
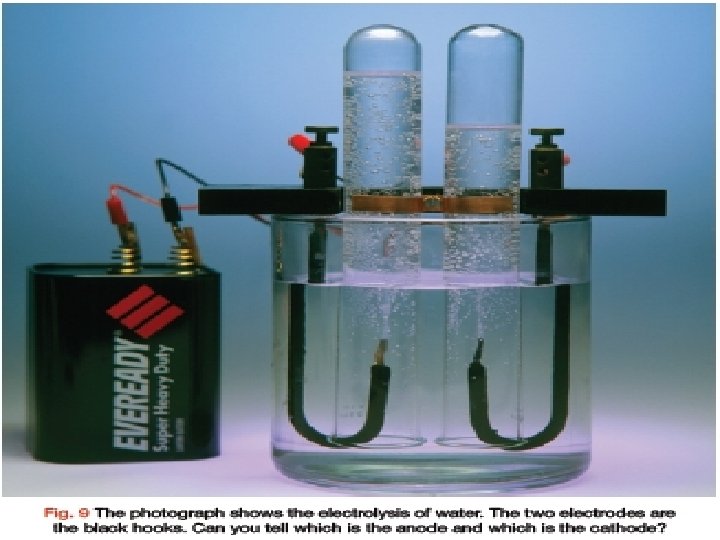
Electrolysis of Water • Electrolysis is the use of electricity to bring about a chemical reaction. • A Hoffman Voltameter is used to pass a current through water causing it to break down into Hydrogen and Oxygen. • Sulfuric acid is added to help the water to conduct electricity. • Platinum electrodes are used.

Hoffman voltameter • Hydrogen gas is collected at the cathode(negative terminal). • Oxygen gas is collected at the anode(positive terminal). • Twice as much Hydrogen as Oxygen is produced. Therefore we represent water by the formula H 2 O.


Breaking down water into its elements , Hydrogen and Oxygen by Electrolysis


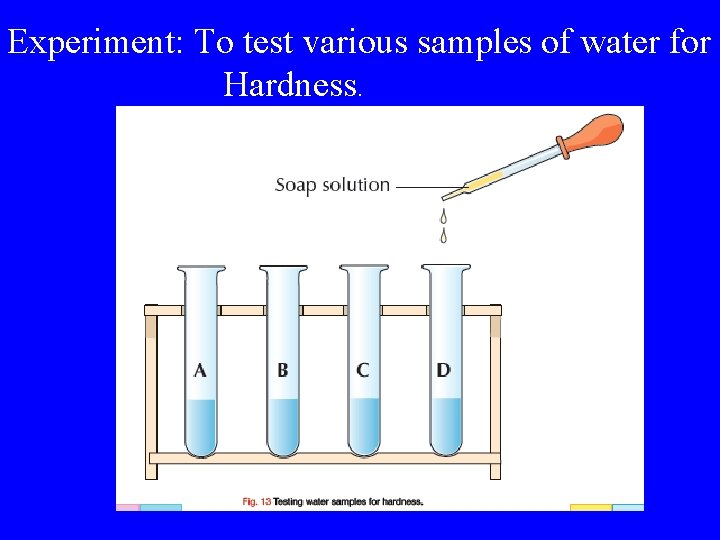
Experiment: To test various samples of water for Hardness.

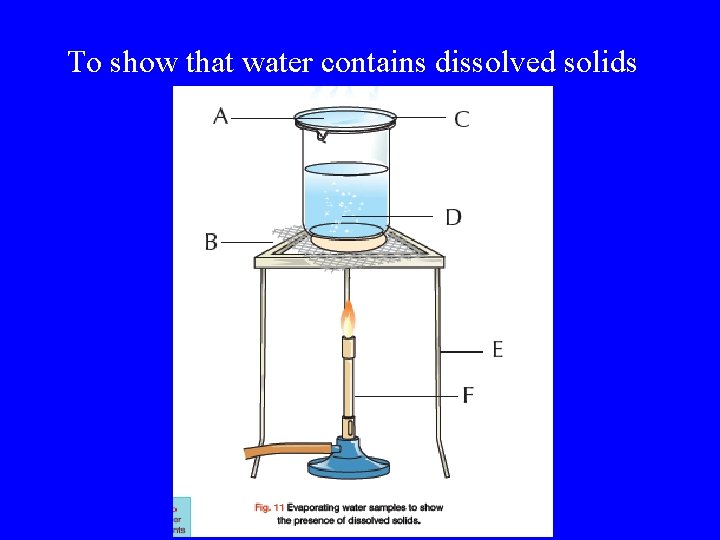
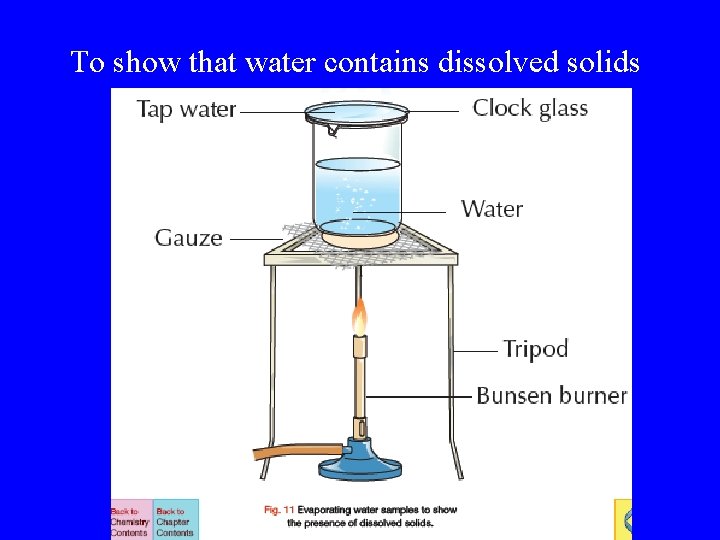
To show that water contains dissolved solids

To show that water contains dissolved solids

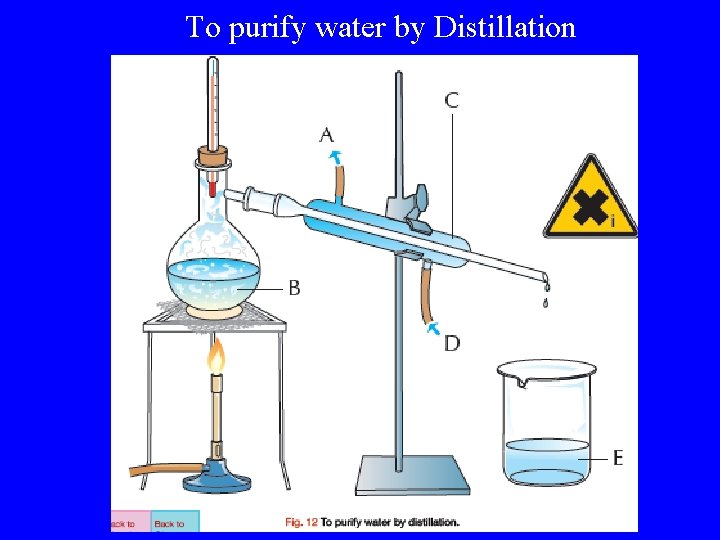
To purify water by Distillation
 Water and water and water water
Water and water and water water Class 8 english chapter 7 water water everywhere
Class 8 english chapter 7 water water everywhere Chapter 9 surface water chapter assessment answer key
Chapter 9 surface water chapter assessment answer key Drinking fountain
Drinking fountain Dock water density
Dock water density Water o water
Water o water 5 divided by 1/4
5 divided by 1/4 Water exchanger
Water exchanger Fresh water meets salt water
Fresh water meets salt water Warm water rises in a lake. cold water descends.
Warm water rises in a lake. cold water descends. Water water everywhere project
Water water everywhere project Water resource
Water resource Unit 11 water water everywhere
Unit 11 water water everywhere Chapter 11 section 4 using water wisely answer key
Chapter 11 section 4 using water wisely answer key We think we are using water wisely because
We think we are using water wisely because Chapter 11-9 the water cycle
Chapter 11-9 the water cycle Water that contains wastes from homes or industry
Water that contains wastes from homes or industry Chapter 7 water the universal solvent
Chapter 7 water the universal solvent Chapter 6 running water and groundwater
Chapter 6 running water and groundwater Chapter 16 section 1 water in the air answer key
Chapter 16 section 1 water in the air answer key Chapter 16 understanding weather answer key
Chapter 16 understanding weather answer key Chapter 15 water and aqueous systems
Chapter 15 water and aqueous systems Chapter 15 water and aqueous systems
Chapter 15 water and aqueous systems Chapter 15 ocean water and ocean life answer key
Chapter 15 ocean water and ocean life answer key On a wet barrel hydrant where is the valve located
On a wet barrel hydrant where is the valve located Chapter 14 water resources
Chapter 14 water resources Chapter 11 section 1 water resources
Chapter 11 section 1 water resources Chapter 11 section 2 water use and management
Chapter 11 section 2 water use and management