WATER CHAPTER 3 1 Water n Water is

























- Slides: 36

WATER CHAPTER 3 -1

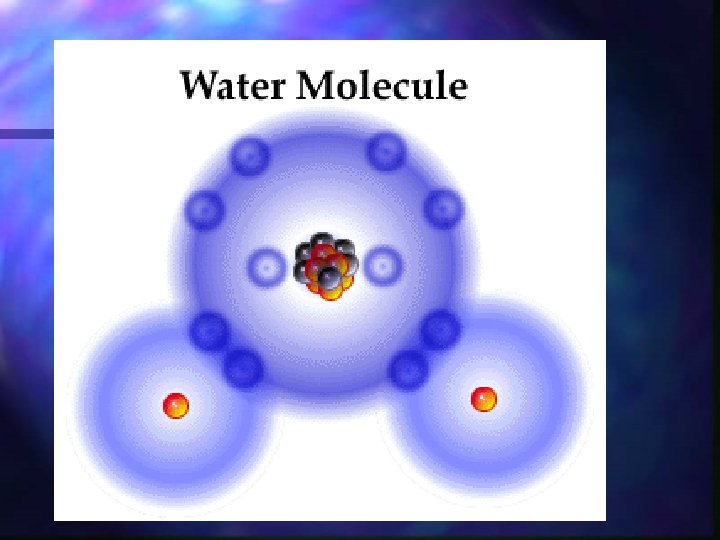
Water n Water is made up of two types of atoms Hydrogen n Oxygen n

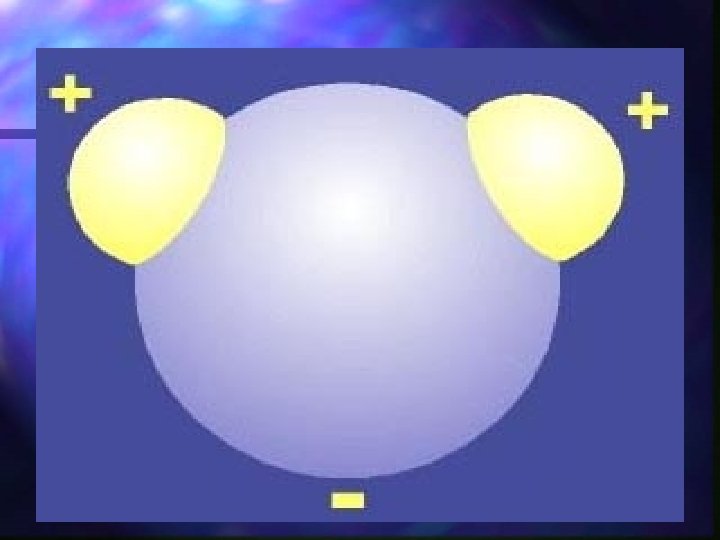
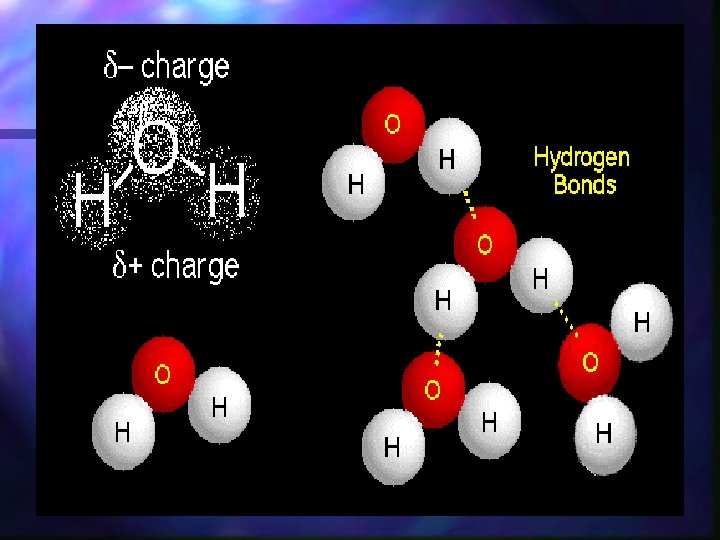
Water is a polar compound n. A polar compound is a molecule with one side having a positive side and the other side with a negative charge. n Water is a polar compound


Polarity n The nucleus of the oxygen atom pulls the shared electrons toward its nucleus and away from the nucleus of the hydrogen atom. n As a result, the electrical charge is unevenly distributed. (polar)


Hydrogen bonding n Hydrogen bonding is the attraction that hold two molecules of water together. n The positive region of one molecule is attracted to the negative region of another.


Capillary Action n Capillary Action- is the property of water that allows it to move upward in a narrow space like a straw. n How a tree moves water.

Cohesion n The attraction of like molecules to each other. n

Adhesion n The attractive force between unlike substances. n

Water is essential for life for many reasons. n Absorbs n Temperature regulation n Great n Solvent Dissolves substances in our body n Body n energy is made up of mostly water Which allows our body to transports substances within the water.

Organic Compounds n An organic compound always contains carbon. A few exceptions are carbon monoxide, carbon dioxide, carbonates, cyanides, cyanates, carbides, and thyocyanates, which are considered inorganic.

Organic Compounds n Carbon is the main atom found in organic compounds. n Carbon atoms can bond together to form rings, straight chains or branched chained molecules.

Energy n Organic molecules contain energy in their bonds n The energy is released when these molecules are broken down

Organic Compounds n Four main types of organic compounds Lipids (fats) n Proteins n Carbohydrates n Nucleic Acids n

Carbohydrates n Organic compounds composed of carbon, hydrogen and oxygen. n Used as energy n The atoms of carbohydrates often form a ring shape. n Examples of carbohydrates are sugar, cellulose and glycogen.

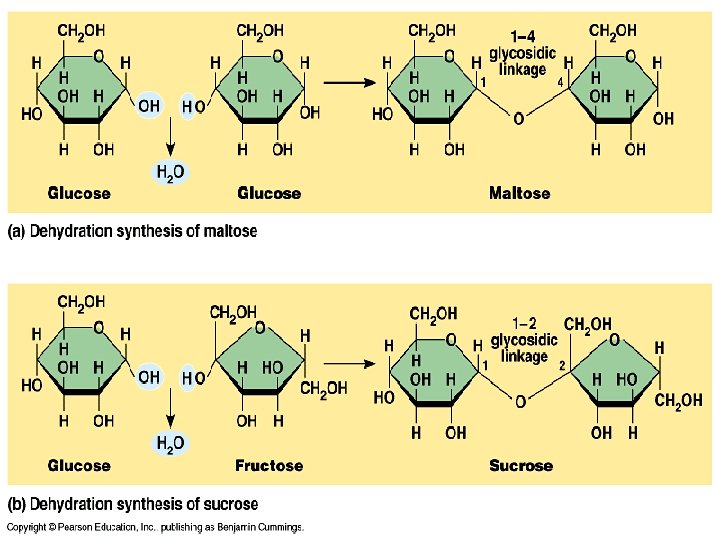
Carbohydrates n Monosaccharide is a simple sugar such as glucose or fructose. n A polysaccharide is a complex molecule made of three or more monosaccharides. Glycogen, starch and cellulose are polysaccharides. n Plants store carbohydrates in the form of starch.

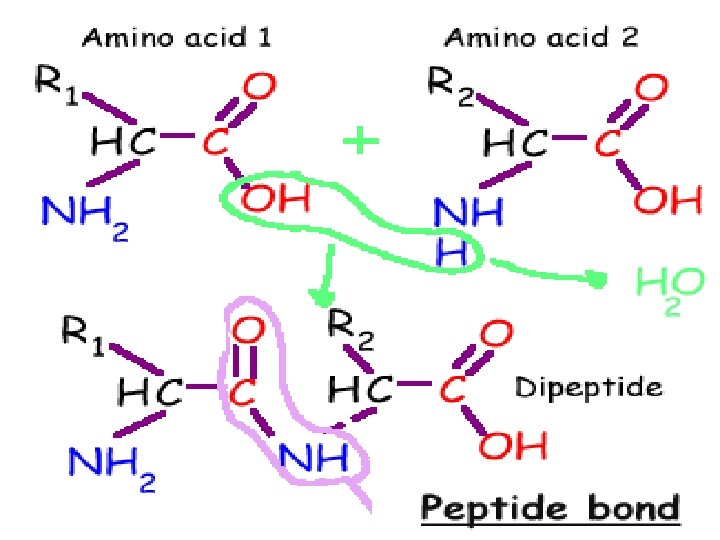
Dehydration Synthesis n When carbohydrates are linked together a molecule of water is given off. n This process is called dehydration synthesis.

Dehydration Reaction

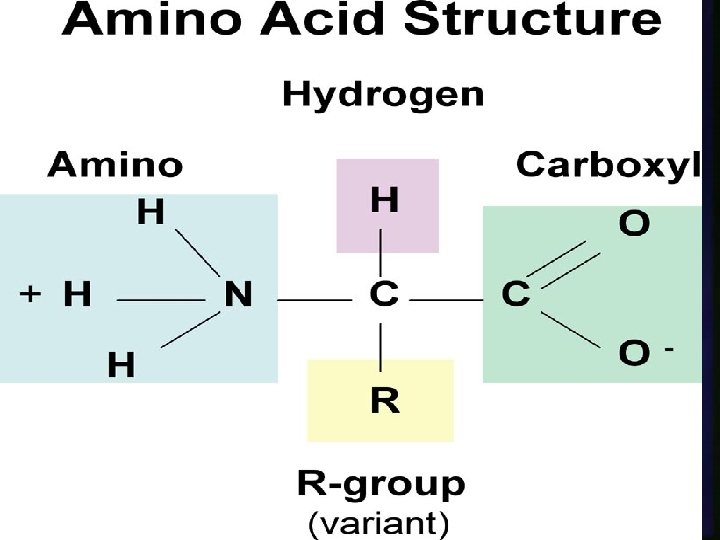
Proteins n The building block of proteins are amino acids. n Amino acids are connected to form large macromolecules called proteins n Proteins often made with branched chains



Enzymes n Proteins are often used to make enzymes n Enzymes things speed up reactions in living

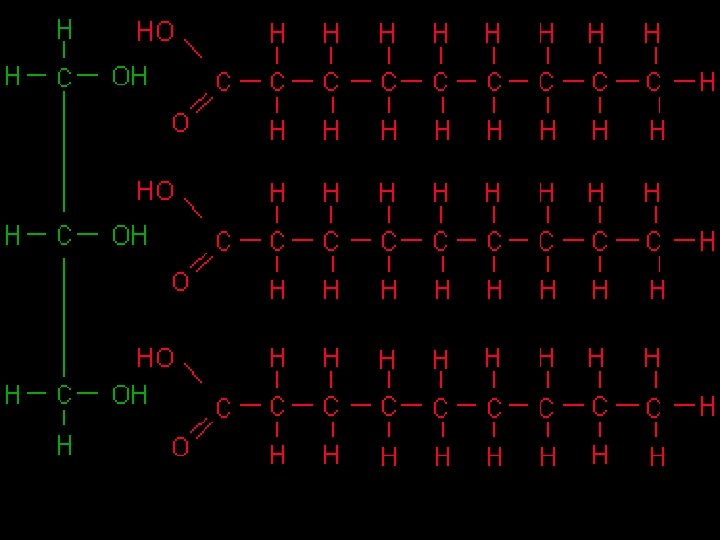
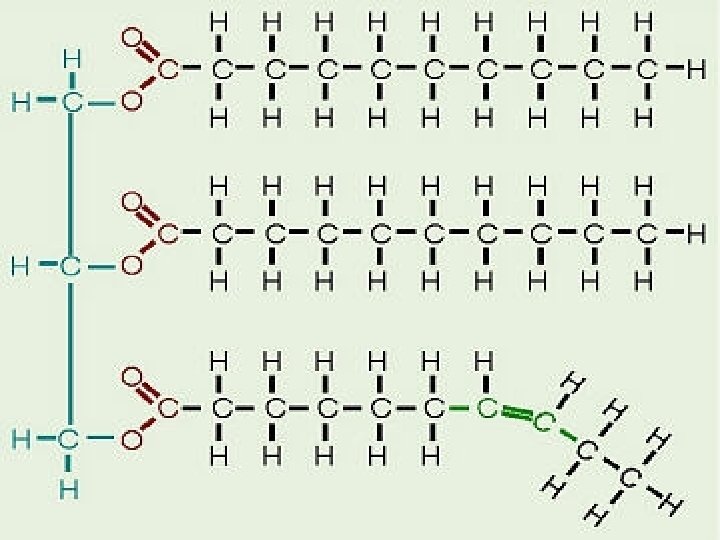
Lipids n Lipids are large nonpolar organic molecules. n Several types of lipids: fatty acids, cholesterol, wax, oil and steroids. n Form long chains of carbons n Fatty acids are unbranched carbon chains that make up most lipids.

Saturated Fats n Saturated with hydrogen's


Unsaturated fats n Are not saturated with hydrogen atoms


Lipids n Lipids contain the most energy per gram than carbohydrates or proteins. n Lipids store energy.

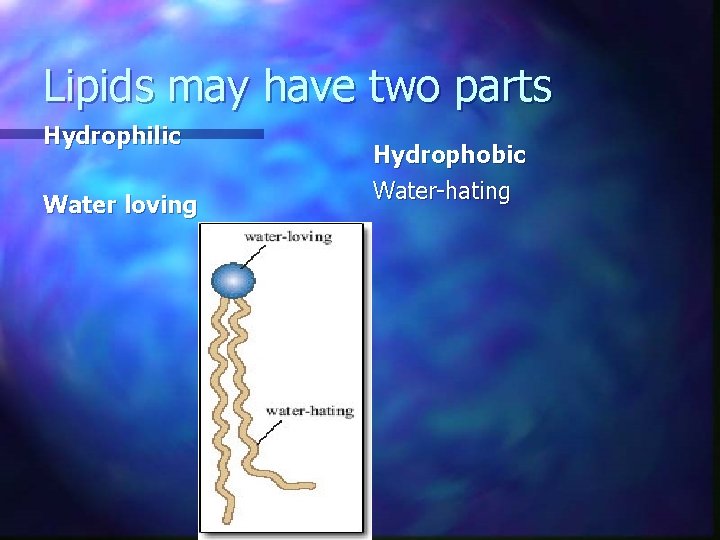
Lipids may have two parts Hydrophilic Water loving Hydrophobic Water-hating

Lipids and Water n Why do water and lipids not mix?

Nucleic Acids n Large complex organic compounds that store important information in living things. n Examples are DNA and RNA n Nucleotides are the building block of nucleic acids (DNA and RNA)

ATP n ATP is a special energy storage nucleotide. n ATP is essentially gasoline for all living things.

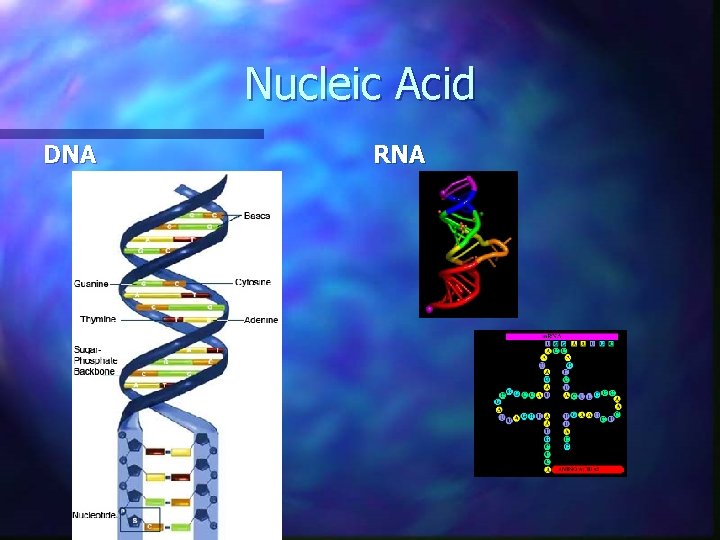
Nucleic Acid DNA RNA

The Six Most Common Atoms In Living Things. n Oxygen n Carbon n Hydrogen n Nitrogen n Calcium n Phosphorus