Voltaic CellsElectrochemical CellsSpontaneous Reactions Electrochemical CellsVoltaic Cells Involve




- Slides: 14

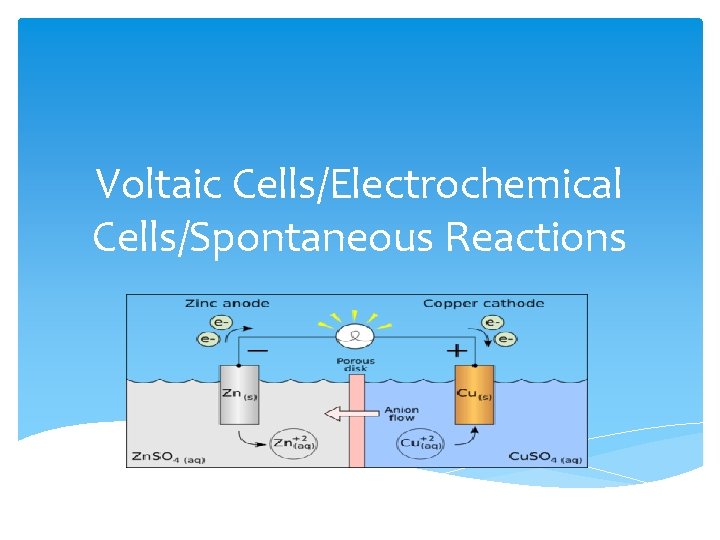
Voltaic Cells/Electrochemical Cells/Spontaneous Reactions

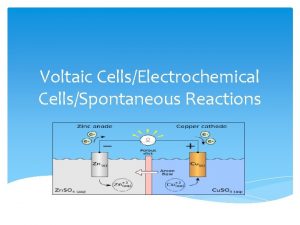
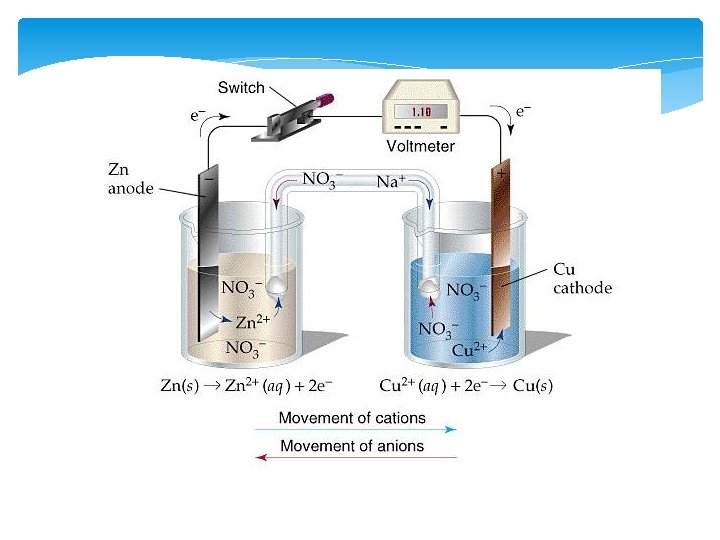
Electrochemical Cells(Voltaic Cells) Involve the conversion of chemical energy into electrical energy Spontaneous reactions take place within the cells A flow of electrons is produced that can be captured to power a device We can use the activity series to see if our cell is operational



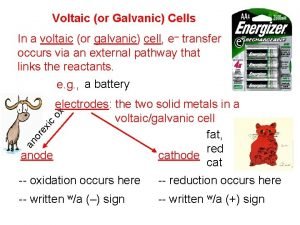
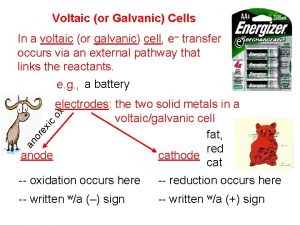
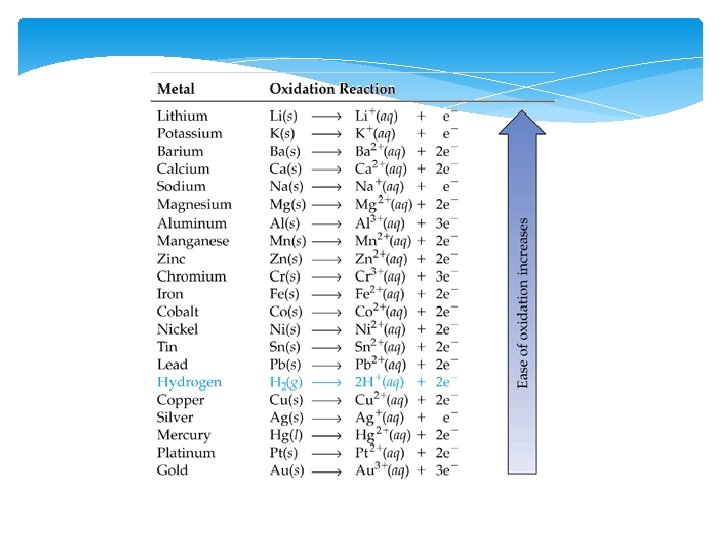
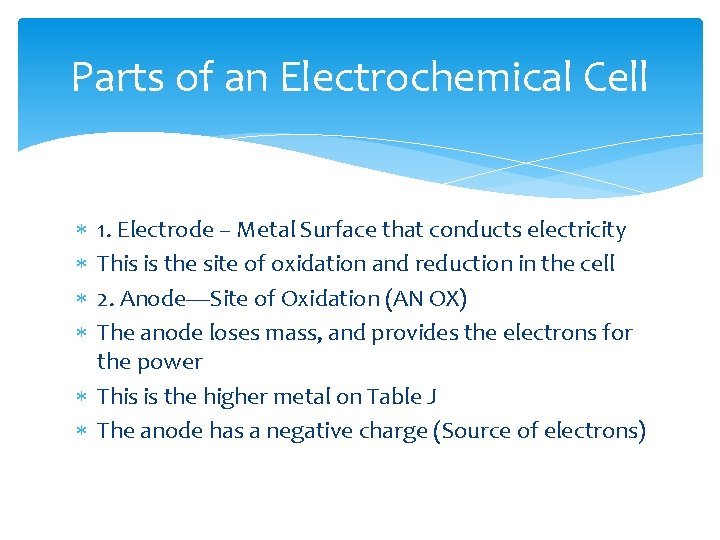
Parts of an Electrochemical Cell 1. Electrode – Metal Surface that conducts electricity This is the site of oxidation and reduction in the cell 2. Anode—Site of Oxidation (AN OX) The anode loses mass, and provides the electrons for the power This is the higher metal on Table J The anode has a negative charge (Source of electrons)

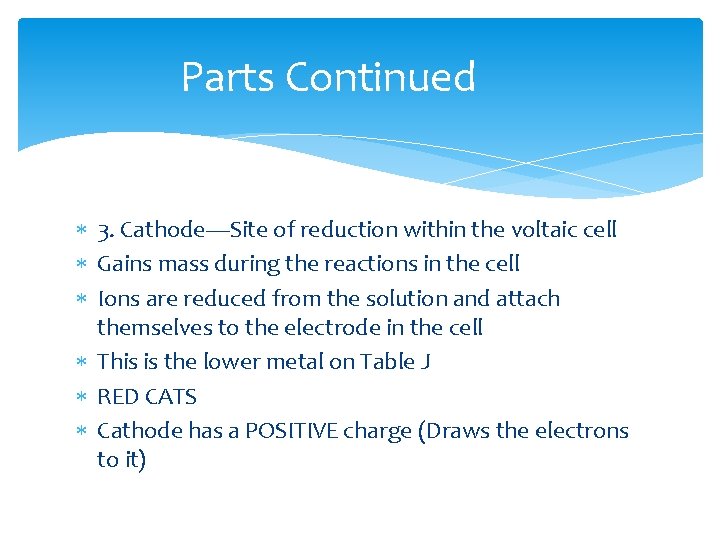
Parts Continued 3. Cathode—Site of reduction within the voltaic cell Gains mass during the reactions in the cell Ions are reduced from the solution and attach themselves to the electrode in the cell This is the lower metal on Table J RED CATS Cathode has a POSITIVE charge (Draws the electrons to it)


Parts Continued 4. Half-Cell—One half of an electrolytic cell set up 5. Salt-Bridge– device that allows for the movement of electrolyte ions Connects the cells to each other to prevent the build up of charge Without the salt-bride the charges build up in the cells and it stops working YOU MUST ALWAYS HAVE A SALT BRIDGE



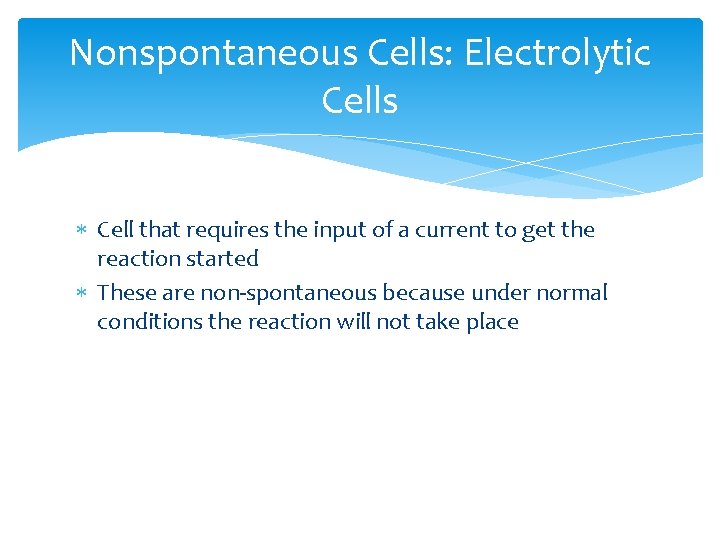
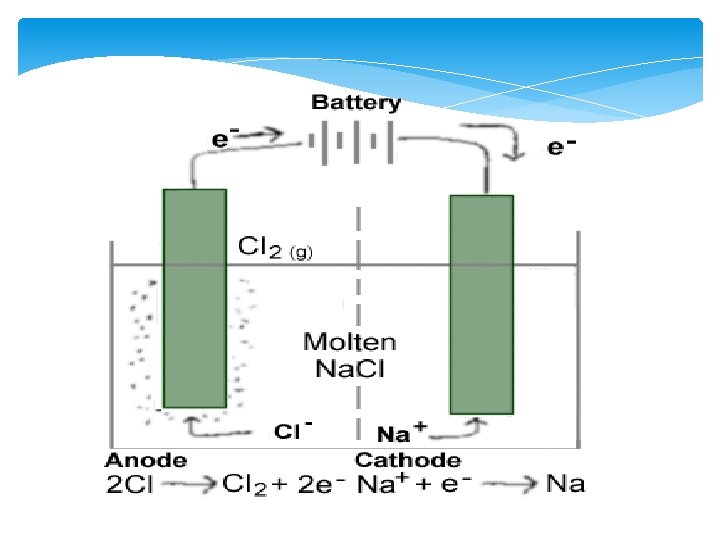
Nonspontaneous Cells: Electrolytic Cells Cell that requires the input of a current to get the reaction started These are non-spontaneous because under normal conditions the reaction will not take place


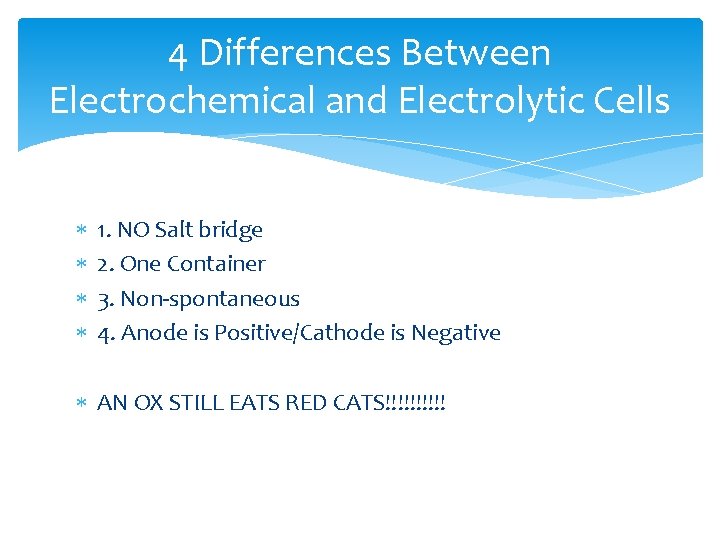
4 Differences Between Electrochemical and Electrolytic Cells 1. NO Salt bridge 2. One Container 3. Non-spontaneous 4. Anode is Positive/Cathode is Negative AN OX STILL EATS RED CATS!!!!!

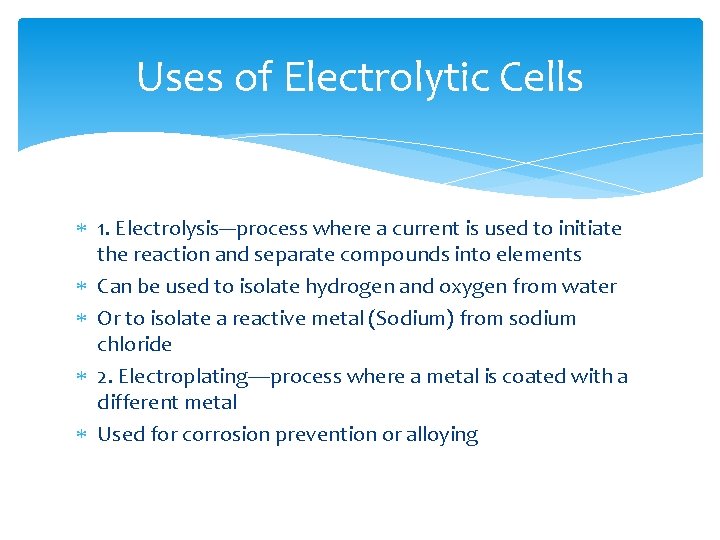
Uses of Electrolytic Cells 1. Electrolysis---process where a current is used to initiate the reaction and separate compounds into elements Can be used to isolate hydrogen and oxygen from water Or to isolate a reactive metal (Sodium) from sodium chloride 2. Electroplating—process where a metal is coated with a different metal Used for corrosion prevention or alloying
 Electrolytic cell
Electrolytic cell Voltaic and galvanic cells
Voltaic and galvanic cells Voltaic cells example #2 worksheet answers
Voltaic cells example #2 worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet How to write redox half reactions
How to write redox half reactions Voltaic cell components
Voltaic cell components Voltaic fundamental
Voltaic fundamental Voltaic aim
Voltaic aim Voltaic cell electron flow
Voltaic cell electron flow Voltaic fundamentals
Voltaic fundamentals Red cat electrochemistry
Red cat electrochemistry Primary voltaic cell
Primary voltaic cell