Using the Law of Requisite Variety LRV to











- Slides: 21

Using the Law of Requisite Variety (LRV) to Manage Selected Federal Programs Presentation at the Policy Studies Organization Dupont Summit December 2, 2016 Morris Bosin - Presenter © 2016 Grant Thornton LLP | All rights reserved

Overview • How do Federal leaders design programs that successfully address the complex and varied needs of their stakeholders? • They design a management approach with a sufficient variety of strategies to match the varied needs of the stakeholders • Insufficient variety does not effectively meet stakeholder needs. Too much variety poses fundamental challenges in managing the program. • Establishing requisite variety requires achieving a delicate balance between over-complexity and over-simplification • You need to collect the right kind of information about the environment in order to design effective strategic responses © 2016 Grant Thornton LLP | All rights reserved

Conversation with My Son – An Ardent Backpacker and Hiker • Me: Would you like to hear about a topic I'm discussing at an upcoming forum? • Son: If you must. • Me: It's called the Law of Requisite Variety • Son: Explain • Me: Blah blah • Son: Let me see if I can explain it in my own terms I'm packing for a one week trip this January on the Appalachian Trail. If I don't pack the right stuff I may get hungry, frozen, lost or attacked by a bear. If I pack for every single possible eventuality the backpack will weigh 90 pounds and I won't be able to stand up. So I have to pack for the important stuff. • Son: Did I get it? • Me: You got it! © 2016 Grant Thornton LLP | All rights reserved

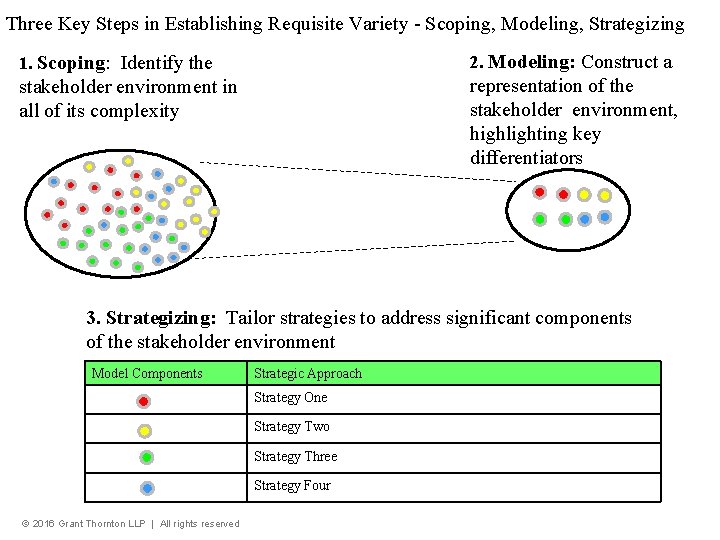
Three Key Steps in Establishing Requisite Variety - Scoping, Modeling, Strategizing 1. Scoping: Identify the 2. Modeling: Construct a stakeholder environment in all of its complexity representation of the stakeholder environment, highlighting key differentiators 3. Strategizing: Tailor strategies to address significant components of the stakeholder environment Model Components Strategic Approach Strategy One Strategy Two Strategy Three Strategy Four © 2016 Grant Thornton LLP | All rights reserved

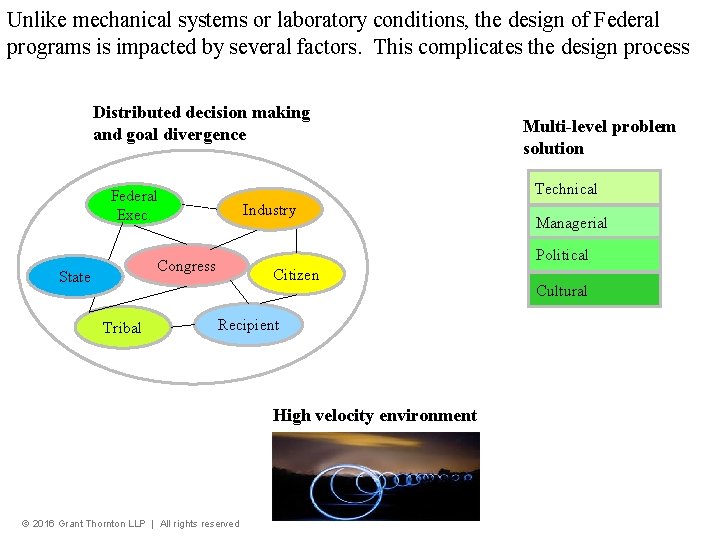
Unlike mechanical systems or laboratory conditions, the design of Federal programs is impacted by several factors. This complicates the design process Distributed decision making and goal divergence Technical Federal Exec. Industry Tribal Managerial Political Congress State Multi-level problem solution Citizen Recipient High velocity environment © 2016 Grant Thornton LLP | All rights reserved Cultural

Applying Requisite Variety to Two Federal Programs FDA – New Drug Review • Responsible for reviewing investigational, new, and generic drug applications from industry to determine their safety and efficacy • Around 100 to 200 new drug applications are reviewed annually, and about 20 -25 new molecular entities are also reviewed (breakthrough drugs) • Although safety and efficacy is their mandate, FDA is surrounded by stakeholders with varied goals and interests Bureau of Indian Education • Oversees educational standards and teaching strategies for 183 BIEowned schools, and Tribal schools, enrolling over 41, 000 students. and Indian colleges and universities • Must respect the sovereignty of Tribal nations and work in the best interest of students' current and future quality of life © 2016 Grant Thornton LLP | All rights reserved

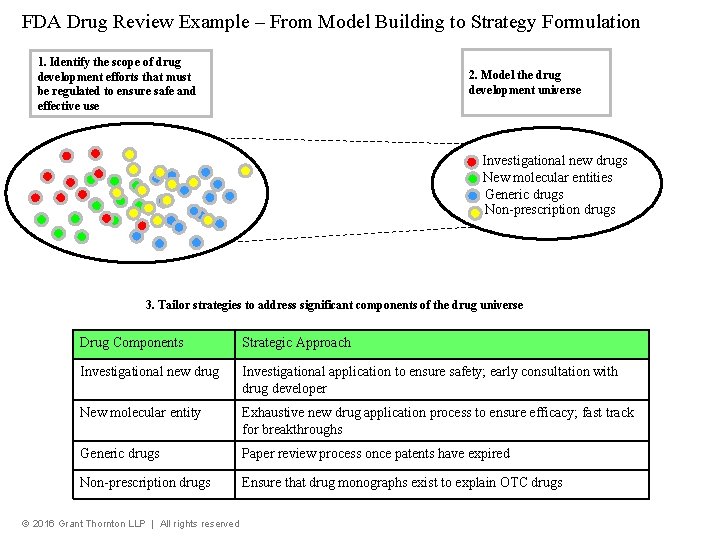
FDA Drug Review Example – From Model Building to Strategy Formulation 1. Identify the scope of drug development efforts that must be regulated to ensure safe and effective use 2. Model the drug development universe Investigational new drugs New molecular entities Generic drugs Non-prescription drugs 3. Tailor strategies to address significant components of the drug universe Drug Components Strategic Approach Investigational new drug Investigational application to ensure safety; early consultation with drug developer New molecular entity Exhaustive new drug application process to ensure efficacy; fast track for breakthroughs Generic drugs Paper review process once patents have expired Non-prescription drugs Ensure that drug monographs exist to explain OTC drugs © 2016 Grant Thornton LLP | All rights reserved

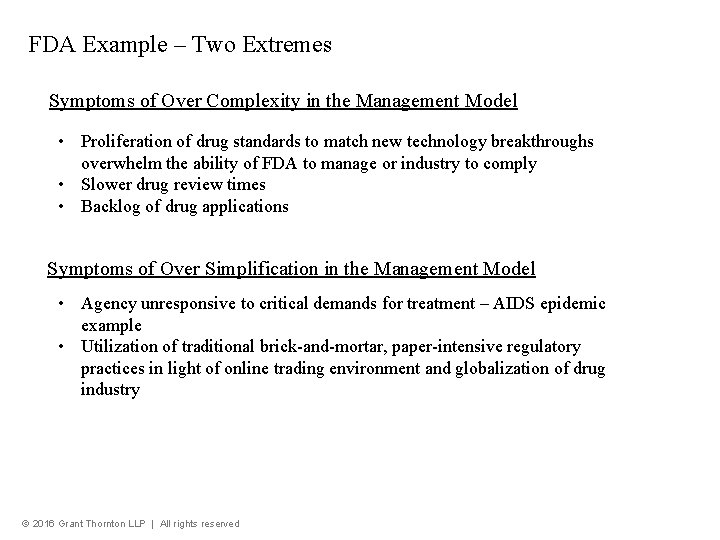
FDA Example – Two Extremes Symptoms of Over Complexity in the Management Model • Proliferation of drug standards to match new technology breakthroughs overwhelm the ability of FDA to manage or industry to comply • Slower drug review times • Backlog of drug applications Symptoms of Over Simplification in the Management Model • Agency unresponsive to critical demands for treatment – AIDS epidemic example • Utilization of traditional brick-and-mortar, paper-intensive regulatory practices in light of online trading environment and globalization of drug industry © 2016 Grant Thornton LLP | All rights reserved

FDA Complexity Challenges and Variety Reducing Solutions Challenge Drug approval decisions are a composite of multi-level perspectives Ø Technical – Science assesses the risk Ø Managerial – Reviewers must consider costs vs benefits in managing the risk Ø Political – Review decisions influenced by views of current Administration Ø Cultural – Lawyers and scientists operate from different paradigms – must arrive at consensus Impact Ø Slower drug reviews Ø Needed medications do not reach patients Variety-Reducing Solution Ø Collaboration – FDA advisory committees provide neutral perspective in drug review decisions Ø Standard protocols define specific roles for scientists, managers and lawyers in the review process Ø User fees for drug review and performance metrics drive accountability and help to galvanize different perspectives to support review decisions Ø FDA attempts to maintain political neutrality © 2016 Grant Thornton LLP | All rights reserved

FDA Complexity Challenges and Variety Reducing Solutions Challenge High velocity technology environment Ø Difficult for drug reviewers to keep pace with changes Ø Difficult for pharmaceutical firms to focus drug development efforts so that they comply with FDA research protocols Impact Ø Slower drug reviews Ø Needed medications do not reach patients Variety-Reducing Solution Ø Technology forecasting - Recruit and train chemists, physicians and pharmacologists based on forecasting emerging trends in new drug development Ø Early consultation - Provide guidance to drug manufacturers early in the drug R&D process to ensure quality applications Ø Online reviews of drug applications to accelerate drug review process Ø Computer simulations to test impact of new molecular entities on the human body; reduces need for time consuming tests using animals © 2016 Grant Thornton LLP | All rights reserved

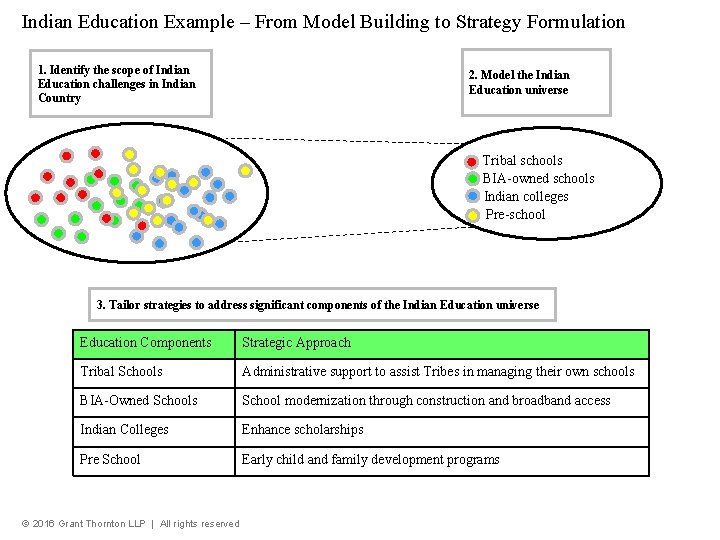
Indian Education Example – From Model Building to Strategy Formulation 1. Identify the scope of Indian Education challenges in Indian Country 2. Model the Indian Education universe Tribal schools BIA-owned schools Indian colleges Pre-school 3. Tailor strategies to address significant components of the Indian Education universe Education Components Strategic Approach Tribal Schools Administrative support to assist Tribes in managing their own schools BIA-Owned Schools School modernization through construction and broadband access Indian Colleges Enhance scholarships Pre School Early child and family development programs © 2016 Grant Thornton LLP | All rights reserved

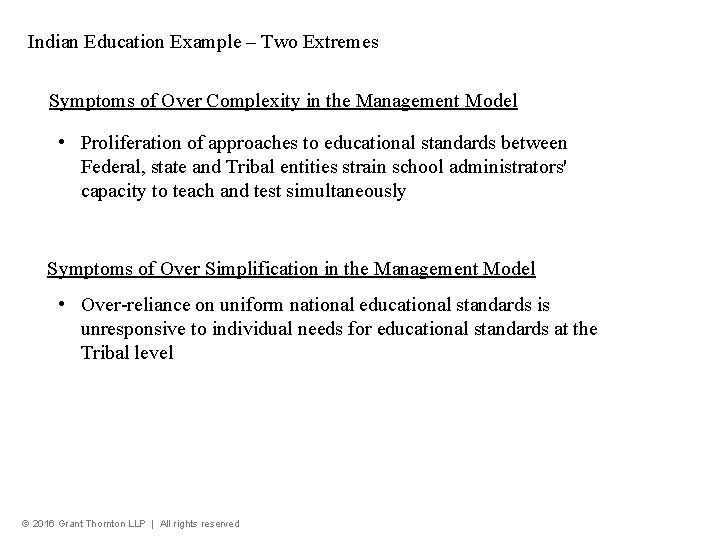
Indian Education Example – Two Extremes Symptoms of Over Complexity in the Management Model • Proliferation of approaches to educational standards between Federal, state and Tribal entities strain school administrators' capacity to teach and test simultaneously Symptoms of Over Simplification in the Management Model • Over-reliance on uniform national educational standards is unresponsive to individual needs for educational standards at the Tribal level © 2016 Grant Thornton LLP | All rights reserved

Indian Education Challenges and Variety Reducing Solutions Challenge Ø Conflicting Federal, state and tribal standards of learning Ø Tension between goals of maintaining cultural integrity and preparing students to succeed in the larger society Impact Ø Testing overwhelms teaching environment, leaving little room for learning Ø Frustrated students who are being tested at levels beyond their capacity Ø Students marginalized between cultures that detract from learning readiness Variety-Reducing Solution Ø Collaborate among governing entities to align standards; have minimum core competencies throughout – establish intergovernmental bodies Ø Align from top down or bottom up? Ø Establish strong leadership at the school level that can mitigate the impact of complex and competing standards; strong leaders are great variety reducers © 2016 Grant Thornton LLP | All rights reserved

Generic Strategies to Reduce Complexity/Absorb Variety Ø Form partnerships that combine complementary capabilities and create synergies "2+2=5" impact § Johnson & Johnson – FDA - FBI – developed a holistic solution to Tylenol tampering Ø Economies of scale § FDA shares laboratory space with other Federal and state agencies to spread fixed costs § Schools spread fixed costs by renting facilities for noneducation purposes Ø Transfer core competencies § Provide guidance to drug manufacturers early in the drug R&D process to ensure quality applications § Establish teacher mentoring programs © 2016 Grant Thornton LLP | All rights reserved

Generic Strategies to Reduce Complexity/Absorb Variety Ø Prioritize risks § Assess student risks; design tailored educational curricula for students at highest risk § Establish fast track reviews for most needed drugs Ø Use information technology § Accelerate drug review by submitting online drug applications § Broaden educational opportunities through online learning; Kahn School example Ø Improve process design § Parallel rather than sequential drug review steps § Team teaching © 2016 Grant Thornton LLP | All rights reserved

Summary • Federal managers instinctively employ LRV in managing complex problems • The challenge is achieving the delicate balance between overcomplexity and over-simplification in the design of an effective management approach • Unlike closed mechanical or human systems, the LRV approach encounters extenuating circumstances when applied in the dynamic open system environment of a Federal program • Strategies are available to assist in reducing complexity and enabling successful problem management…if not resolution © 2016 Grant Thornton LLP | All rights reserved

Appendix © 2016 Grant Thornton LLP | All rights reserved

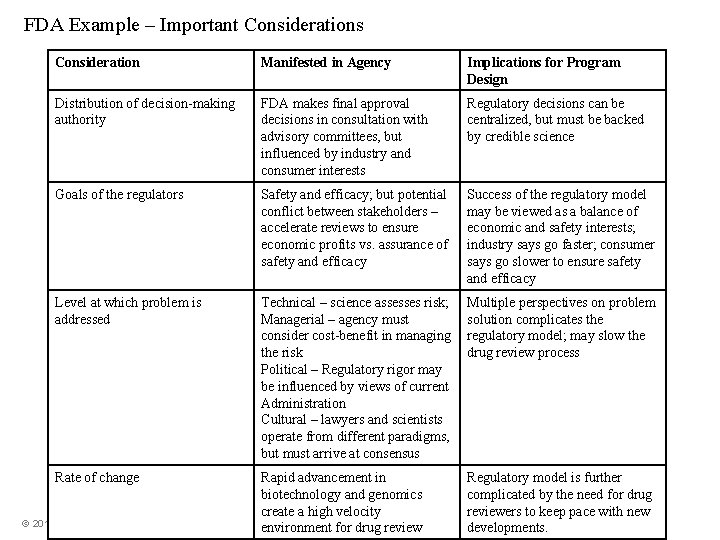
FDA Example – Important Considerations Consideration Manifested in Agency Implications for Program Design Distribution of decision-making authority FDA makes final approval decisions in consultation with advisory committees, but influenced by industry and consumer interests Regulatory decisions can be centralized, but must be backed by credible science Goals of the regulators Safety and efficacy; but potential conflict between stakeholders – accelerate reviews to ensure economic profits vs. assurance of safety and efficacy Success of the regulatory model may be viewed as a balance of economic and safety interests; industry says go faster; consumer says go slower to ensure safety and efficacy Level at which problem is addressed Technical – science assesses risk; Managerial – agency must consider cost-benefit in managing the risk Political – Regulatory rigor may be influenced by views of current Administration Cultural – lawyers and scientists operate from different paradigms, but must arrive at consensus Multiple perspectives on problem solution complicates the regulatory model; may slow the drug review process Rate of change Rapid advancement in biotechnology and genomics create a high velocity environment for drug review Regulatory model is further complicated by the need for drug reviewers to keep pace with new developments. © 2016 Grant Thornton LLP | All rights reserved

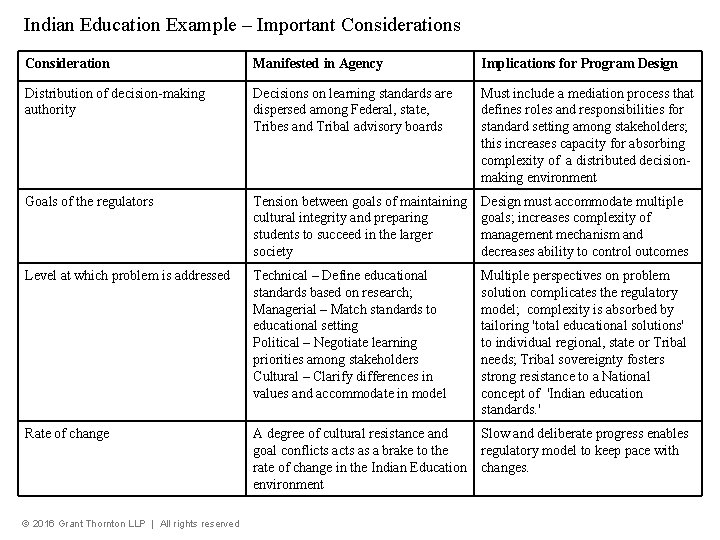
Indian Education Example – Important Considerations Consideration Manifested in Agency Implications for Program Design Distribution of decision-making authority Decisions on learning standards are dispersed among Federal, state, Tribes and Tribal advisory boards Must include a mediation process that defines roles and responsibilities for standard setting among stakeholders; this increases capacity for absorbing complexity of a distributed decisionmaking environment Goals of the regulators Tension between goals of maintaining cultural integrity and preparing students to succeed in the larger society Design must accommodate multiple goals; increases complexity of management mechanism and decreases ability to control outcomes Level at which problem is addressed Technical – Define educational standards based on research; Managerial – Match standards to educational setting Political – Negotiate learning priorities among stakeholders Cultural – Clarify differences in values and accommodate in model Multiple perspectives on problem solution complicates the regulatory model; complexity is absorbed by tailoring 'total educational solutions' to individual regional, state or Tribal needs; Tribal sovereignty fosters strong resistance to a National concept of 'Indian education standards. ' Rate of change A degree of cultural resistance and goal conflicts as a brake to the rate of change in the Indian Education environment Slow and deliberate progress enables regulatory model to keep pace with changes. © 2016 Grant Thornton LLP | All rights reserved

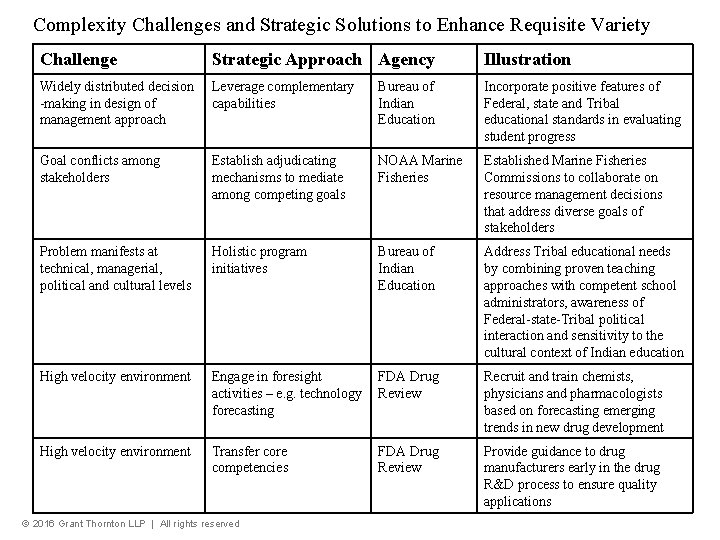
Complexity Challenges and Strategic Solutions to Enhance Requisite Variety Challenge Strategic Approach Agency Illustration Widely distributed decision -making in design of management approach Leverage complementary capabilities Bureau of Indian Education Incorporate positive features of Federal, state and Tribal educational standards in evaluating student progress Goal conflicts among stakeholders Establish adjudicating mechanisms to mediate among competing goals NOAA Marine Fisheries Established Marine Fisheries Commissions to collaborate on resource management decisions that address diverse goals of stakeholders Problem manifests at technical, managerial, political and cultural levels Holistic program initiatives Bureau of Indian Education Address Tribal educational needs by combining proven teaching approaches with competent school administrators, awareness of Federal-state-Tribal political interaction and sensitivity to the cultural context of Indian education High velocity environment Engage in foresight activities – e. g. technology forecasting FDA Drug Review Recruit and train chemists, physicians and pharmacologists based on forecasting emerging trends in new drug development High velocity environment Transfer core competencies FDA Drug Review Provide guidance to drug manufacturers early in the drug R&D process to ensure quality applications © 2016 Grant Thornton LLP | All rights reserved

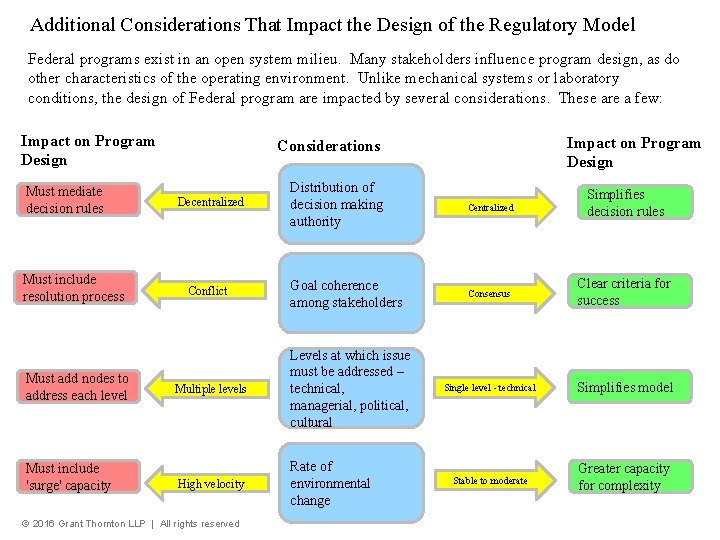
Additional Considerations That Impact the Design of the Regulatory Model Federal programs exist in an open system milieu. Many stakeholders influence program design, as do other characteristics of the operating environment. Unlike mechanical systems or laboratory conditions, the design of Federal program are impacted by several considerations. These are a few: Impact on Program Design Must mediate decision rules Must include resolution process Must add nodes to address each level Must include 'surge' capacity Impact on Program Design Considerations Decentralized Conflict Distribution of decision making authority Centralized Simplifies decision rules Goal coherence among stakeholders Consensus Clear criteria for success Single level - technical Simplifies model Stable to moderate Greater capacity for complexity Multiple levels Levels at which issue must be addressed – technical, managerial, political, cultural High velocity Rate of environmental change © 2016 Grant Thornton LLP | All rights reserved
 Dr anbari
Dr anbari Prerequisite programs for haccp
Prerequisite programs for haccp Dorothea e orem
Dorothea e orem Self care requisite
Self care requisite Requisite pro
Requisite pro Requisite skills definition
Requisite skills definition Requisite humility definition
Requisite humility definition Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Boyles law
Boyles law Boyle's law charles law avogadro's law
Boyle's law charles law avogadro's law Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng