Using the Clicker If you have a clicker







- Slides: 27

Using the “Clicker” If you have a clicker now, and did not do this last time, please enter your ID in your clicker. First, turn on your clicker by sliding the power switch, on the left, up. Next, store your student number in the clicker. You only have to do this once. Press the * button to enter the setup menu. Press the up arrow button to get to ID Press the big green arrow key Press the T button, then the up arrow to get a U Enter the rest of your BU ID. Press the big green arrow key.

Post-test on Web. CT; Assignment 12 Don’t forget to submit the post-test! Also, Assignment 12 on Web. Assign is optional (although you will be responsible for this material on the final exam), but you can use your score on the assignment to replace your lowest assignment score (out of 20 points) of the semester. Exam location (Monday Dec. 17 th from 6 -9 pm) 8 am and 6 pm classes: MOR 101 2 pm class: SCI 107

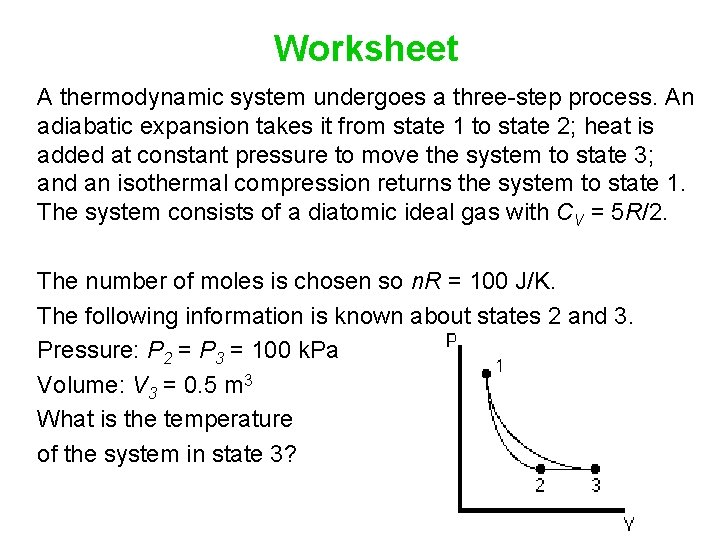
Worksheet A thermodynamic system undergoes a three-step process. An adiabatic expansion takes it from state 1 to state 2; heat is added at constant pressure to move the system to state 3; and an isothermal compression returns the system to state 1. The system consists of a diatomic ideal gas with CV = 5 R/2. The number of moles is chosen so n. R = 100 J/K. The following information is known about states 2 and 3. Pressure: P 2 = P 3 = 100 k. Pa Volume: V 3 = 0. 5 m 3 What is the temperature of the system in state 3?

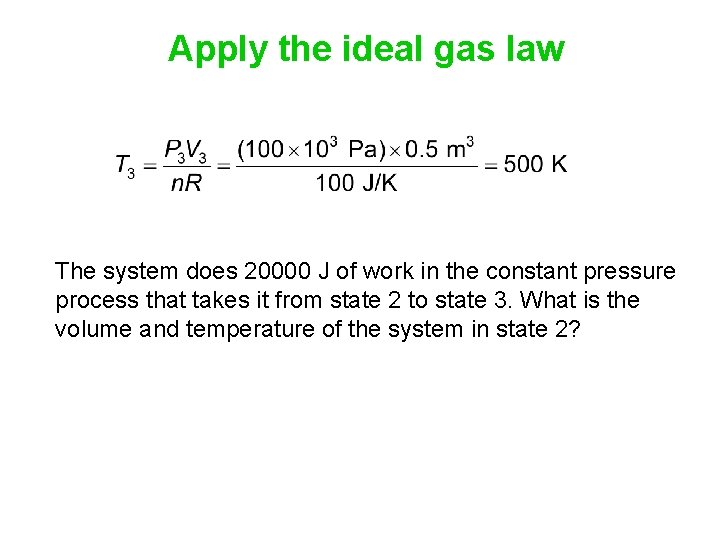
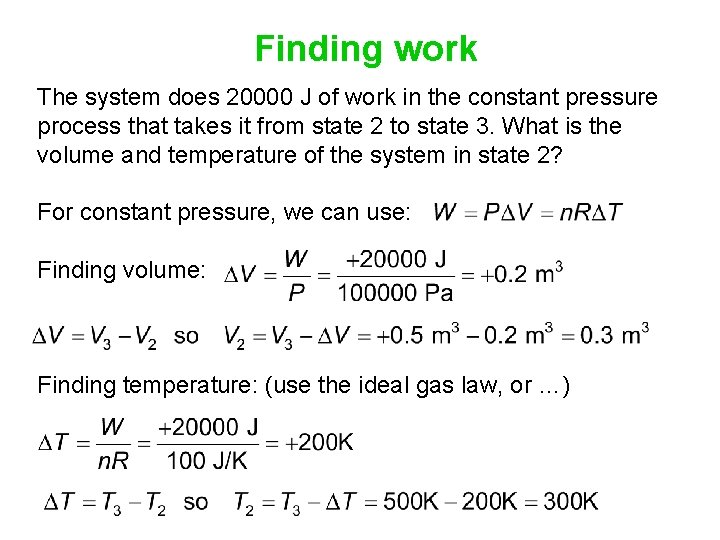
Apply the ideal gas law The system does 20000 J of work in the constant pressure process that takes it from state 2 to state 3. What is the volume and temperature of the system in state 2?

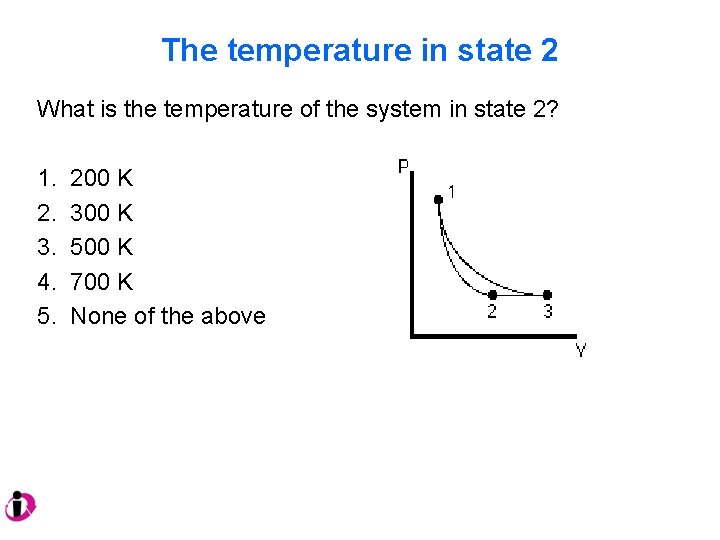
The temperature in state 2 What is the temperature of the system in state 2? 1. 2. 3. 4. 5. 200 K 300 K 500 K 700 K None of the above

Finding work The system does 20000 J of work in the constant pressure process that takes it from state 2 to state 3. What is the volume and temperature of the system in state 2? For constant pressure, we can use: Finding volume: Finding temperature: (use the ideal gas law, or …)

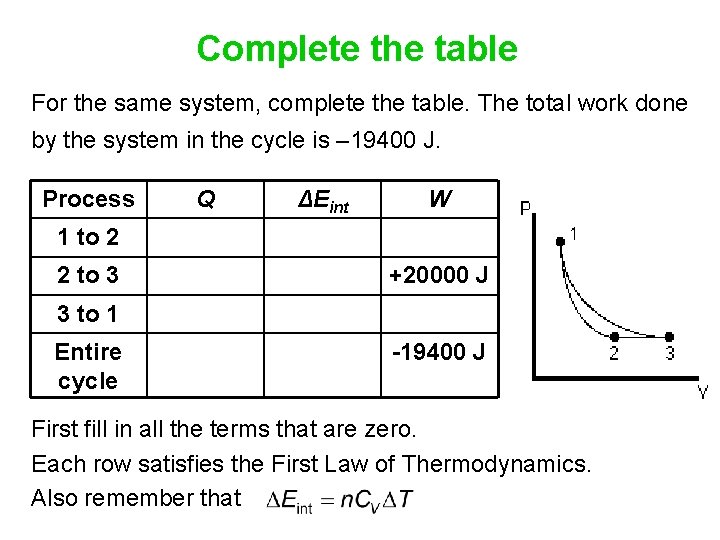
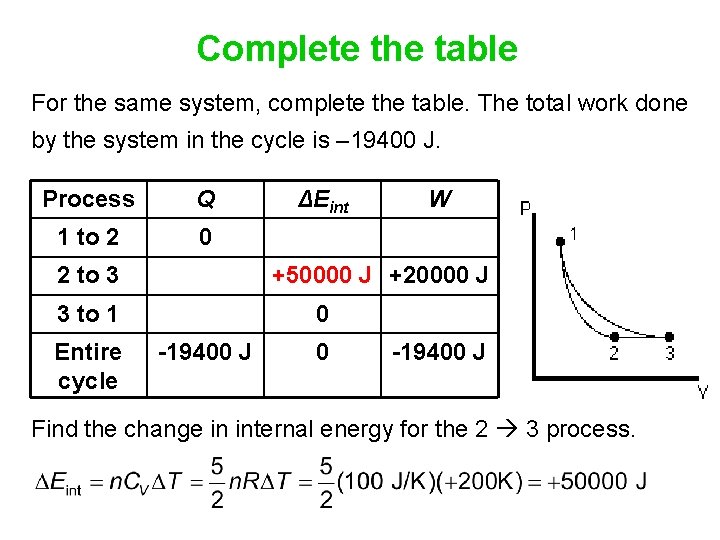
Complete the table For the same system, complete the table. The total work done by the system in the cycle is – 19400 J. Process Q ΔEint W 1 to 2 2 to 3 +20000 J 3 to 1 Entire cycle -19400 J First fill in all the terms that are zero. Each row satisfies the First Law of Thermodynamics. Also remember that

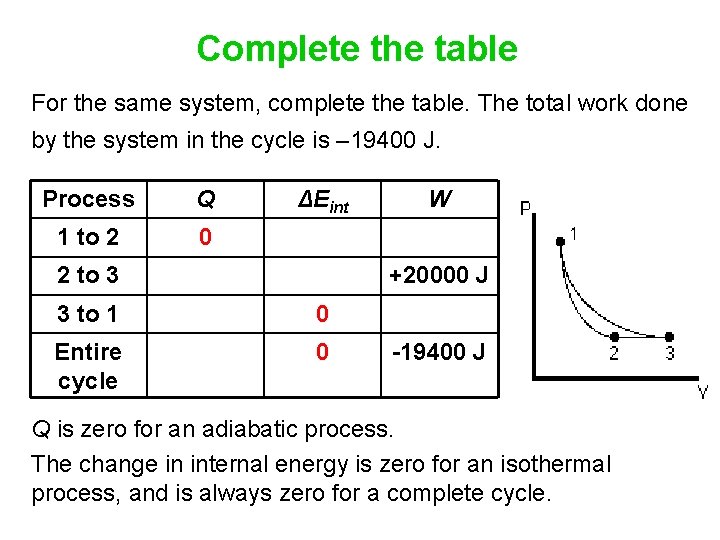
Complete the table For the same system, complete the table. The total work done by the system in the cycle is – 19400 J. Process Q 1 to 2 0 ΔEint 2 to 3 W +20000 J 3 to 1 0 Entire cycle 0 -19400 J Q is zero for an adiabatic process. The change in internal energy is zero for an isothermal process, and is always zero for a complete cycle.

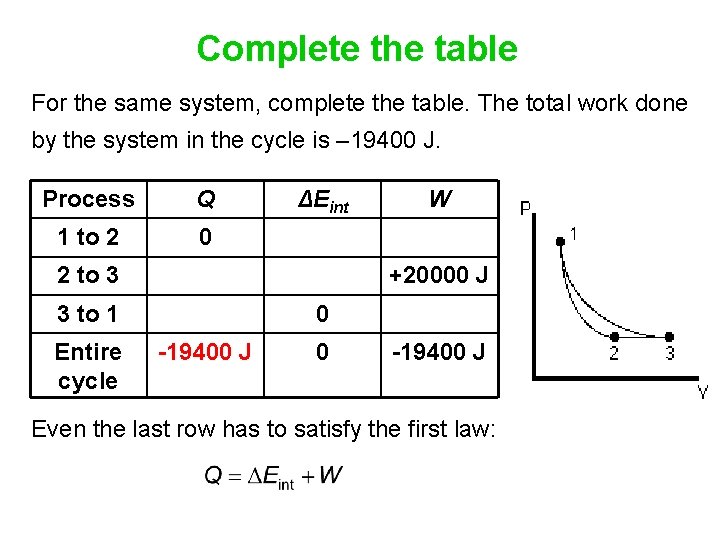
Complete the table For the same system, complete the table. The total work done by the system in the cycle is – 19400 J. Process Q 1 to 2 0 ΔEint 2 to 3 +20000 J 3 to 1 Entire cycle W 0 -19400 J Even the last row has to satisfy the first law:

Complete the table For the same system, complete the table. The total work done by the system in the cycle is – 19400 J. Process Q 1 to 2 0 2 to 3 W +50000 J +20000 J 3 to 1 Entire cycle ΔEint 0 -19400 J Find the change in internal energy for the 2 3 process.

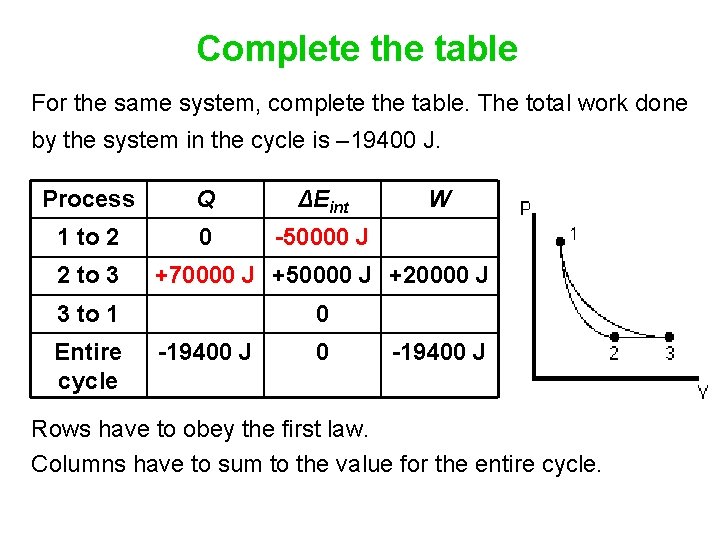
Complete the table For the same system, complete the table. The total work done by the system in the cycle is – 19400 J. Process Q ΔEint 1 to 2 0 -50000 J W 2 to 3 +70000 J +50000 J +20000 J 3 to 1 0 Entire cycle -19400 J 0 -19400 J Rows have to obey the first law. Columns have to sum to the value for the entire cycle.

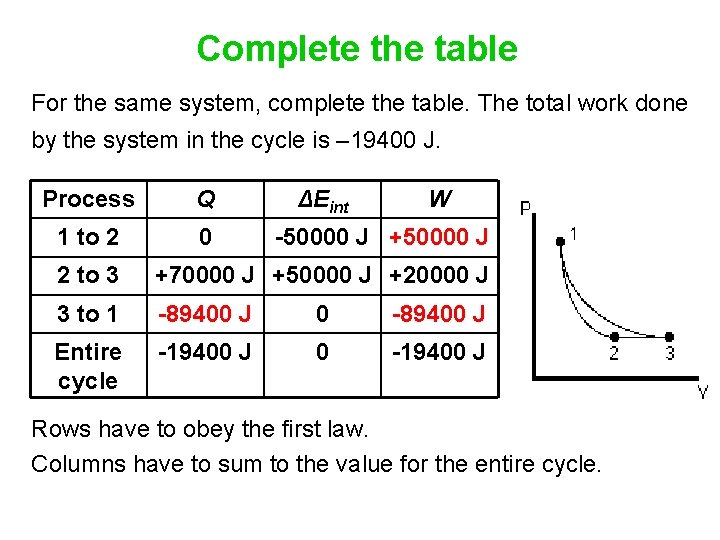
Complete the table For the same system, complete the table. The total work done by the system in the cycle is – 19400 J. Process Q 1 to 2 0 ΔEint W -50000 J +50000 J 2 to 3 +70000 J +50000 J +20000 J 3 to 1 -89400 J 0 -89400 J Entire cycle -19400 J 0 -19400 J Rows have to obey the first law. Columns have to sum to the value for the entire cycle.

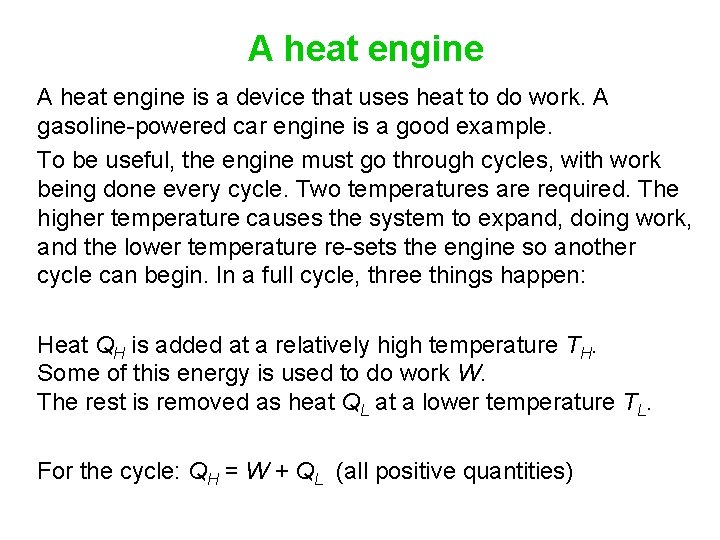
A heat engine is a device that uses heat to do work. A gasoline-powered car engine is a good example. To be useful, the engine must go through cycles, with work being done every cycle. Two temperatures are required. The higher temperature causes the system to expand, doing work, and the lower temperature re-sets the engine so another cycle can begin. In a full cycle, three things happen: Heat QH is added at a relatively high temperature TH. Some of this energy is used to do work W. The rest is removed as heat QL at a lower temperature TL. For the cycle: QH = W + QL (all positive quantities)

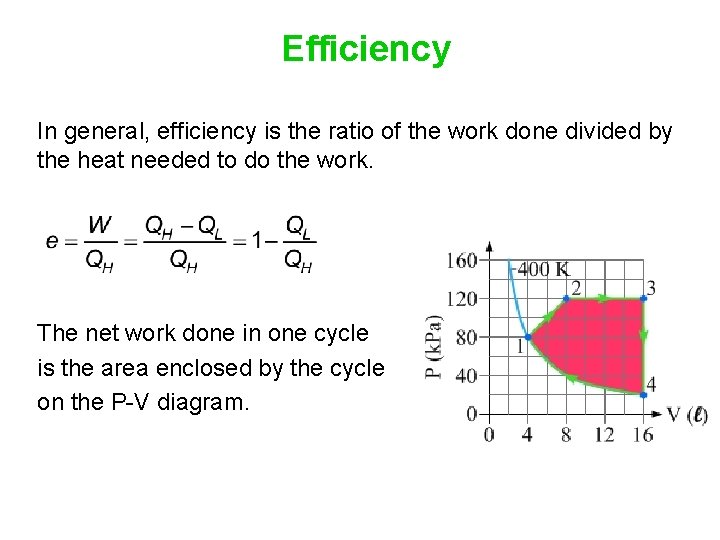
Efficiency In general, efficiency is the ratio of the work done divided by the heat needed to do the work. The net work done in one cycle is the area enclosed by the cycle on the P-V diagram.

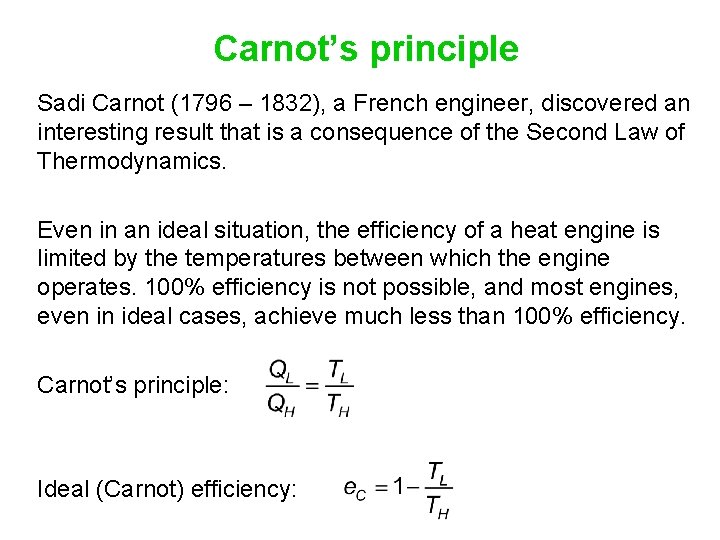
Carnot’s principle Sadi Carnot (1796 – 1832), a French engineer, discovered an interesting result that is a consequence of the Second Law of Thermodynamics. Even in an ideal situation, the efficiency of a heat engine is limited by the temperatures between which the engine operates. 100% efficiency is not possible, and most engines, even in ideal cases, achieve much less than 100% efficiency. Carnot’s principle: Ideal (Carnot) efficiency:

A refrigerator If you had a refrigerator in a closed, well-insulated room and you left the fridge door open for a long time, what would happen to the temperature in the room? 1. It would increase 2. It would decrease 3. It would stay the same

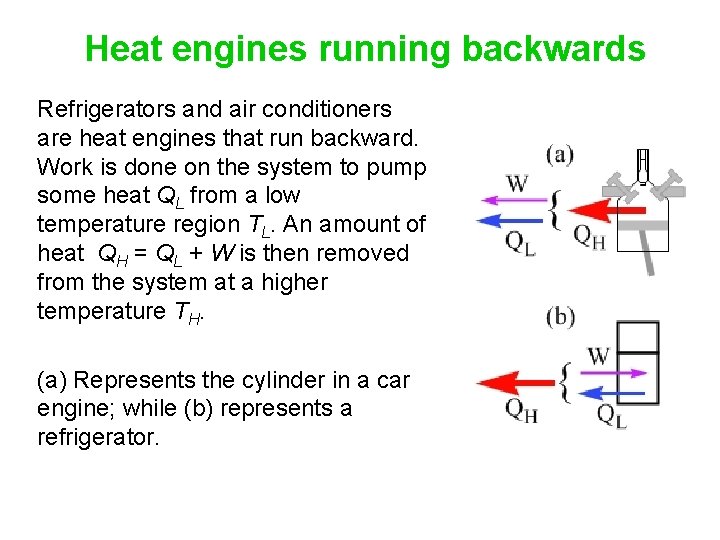
Heat engines running backwards Refrigerators and air conditioners are heat engines that run backward. Work is done on the system to pump some heat QL from a low temperature region TL. An amount of heat QH = QL + W is then removed from the system at a higher temperature TH. (a) Represents the cylinder in a car engine; while (b) represents a refrigerator.

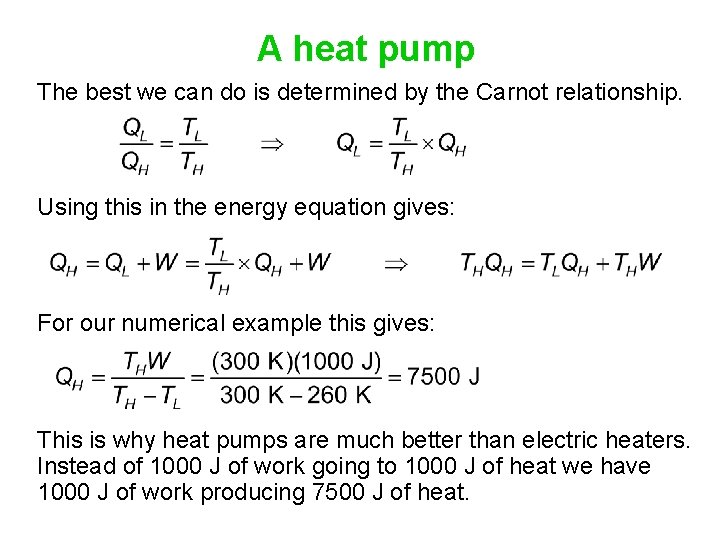
A heat pump If you heat your home using electric heat, 1000 J of electrical energy can be transformed into 1000 J of heat. An alternate way of heating is to use a heat pump, which extracts heat from a lower-temperature region (outside the house) and transfers it to the higher-temperature region (inside the house). Let's say the work done in the process is 1000 J, and the temperatures are TH = 27°C = 300 K and TL = -13 °C = 260 K. What is the maximum amount of heat that can be transferred into the house? 1. Something less than 1000 J 2. 1000 J 3. Something more than 1000 J

A heat pump The best we can do is determined by the Carnot relationship. Using this in the energy equation gives: For our numerical example this gives: This is why heat pumps are much better than electric heaters. Instead of 1000 J of work going to 1000 J of heat we have 1000 J of work producing 7500 J of heat.

Entropy is in some sense a measure of disorder. The symbol for entropy is S, and the units are J/K. A container of ideal gas has an entropy value, just as it has a pressure, a volume, and a temperature. Unlike P, V, and T, which are quite easy to measure, the entropy of a system is difficult to calculate. On the other, a change in entropy is easy to determine.

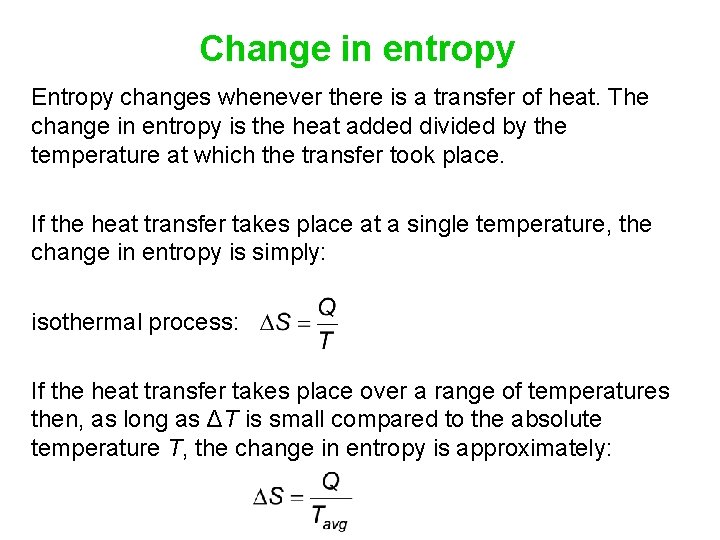
Change in entropy Entropy changes whenever there is a transfer of heat. The change in entropy is the heat added divided by the temperature at which the transfer took place. If the heat transfer takes place at a single temperature, the change in entropy is simply: isothermal process: If the heat transfer takes place over a range of temperatures then, as long as ΔT is small compared to the absolute temperature T, the change in entropy is approximately:

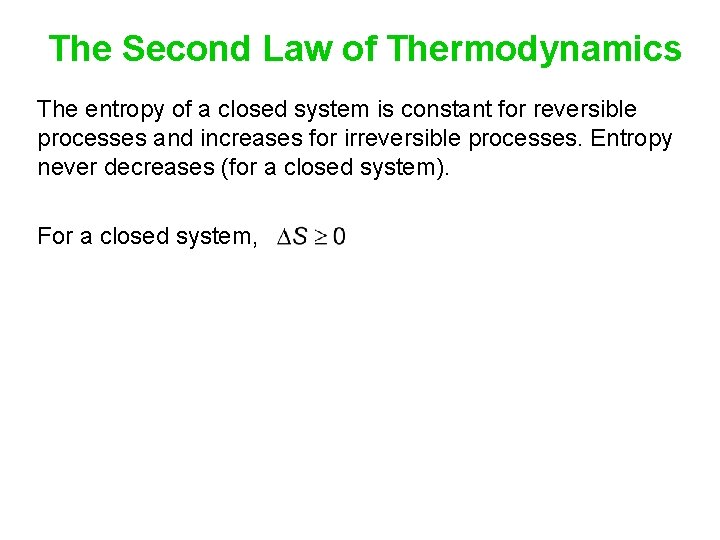
The Second Law of Thermodynamics The entropy of a closed system is constant for reversible processes and increases for irreversible processes. Entropy never decreases (for a closed system). For a closed system,

Dropping a glass You drop a glass of milk and the glass smashes into 27 pieces and milk spills all over the floor. If you videotaped this and ran the film backwards, it would be obvious to you that the film was running backwards. Why? The process violates: 1. The Law of Conservation of Energy 2. The Law of Conservation of Momentum 3. The Second Law of Thermodynamics 4. All of the above

Entropy: time’s arrow In the process of smashing the glass of milk, both energy and momentum are conserved. However, the entropy is increased The direction of time is the direction of increasing entropy. Reversible and irreversible processes In an irreversible process, the entropy of a closed system increases. In a reversible process, the entropy stays the same.

Reversible or not? You have two styrofoam containers of water. Each holds 1 kg of water. In one the water temperature is 17°C, while in the other it is 37°C. The colder water is then poured into the warmer water, and the system is allowed to come to equilibrium. Is this process reversible or irreversible? 1. Reversible 2. Irreversible

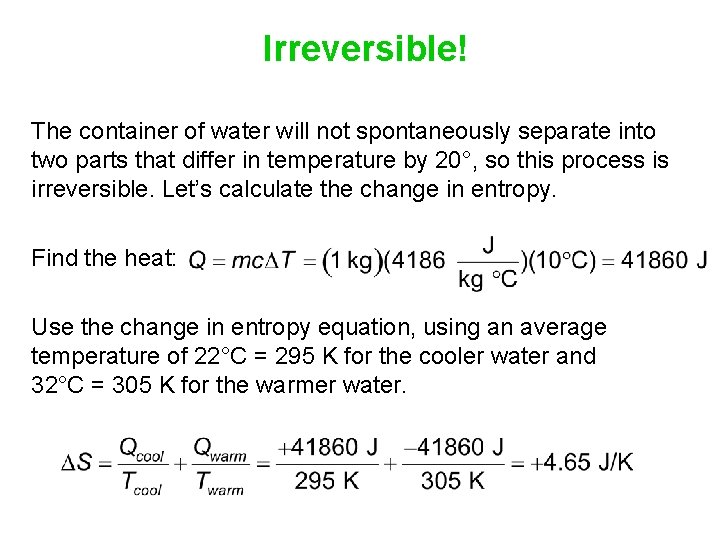
Irreversible! The container of water will not spontaneously separate into two parts that differ in temperature by 20°, so this process is irreversible. Let’s calculate the change in entropy. Find the heat: Use the change in entropy equation, using an average temperature of 22°C = 295 K for the cooler water and 32°C = 305 K for the warmer water.

Whiteboard