Get a clicker please Semester Exam Review Please









































- Slides: 63

Get a clicker please Semester Exam Review

Please make your selection �The smallest particle of an element that retains the properties of that element is a(n) ____. �A proton �B atom �C electron �D neutron

Please make your selection How many valence electrons are in an atom of silicon? A) 4 B) 2 C) 3 D) 5

Please make your selection What is the formula of the ion formed when potassium achieves noble-gas electron configuration? A) K+ B) KC) K 2+ D) K 2 -

Please make your selection Two features that distinguish matter are A) B) C) D) Mass and velocity Weight and velocity Mass and volume Weight and volume

Please make your selection �A horizontal row of the periodic table is usually referred to as a �A family. �B period. �C group. �D property.

Please make your selection The branch of chemistry that includes the study of materials and processes that occur in living things is A. B. C. D. Organic chemistry Physical chemistry Analytical chemistry Biochemistry

Please make your selection The study of matter and the changes in matter best describes the science of A) B) C) D) Biology Physics Microbiology Chemistry

Please make your selection Which of the following is a heterogeneous mixture? A) soil B) air C) steel D) salt water

Please make your selection �What does the number 14 in the name carbon-14 represent? �A the mass number �B the atomic number �C twice the number of protons �D the sum of the protons and electrons

Please make your selection Chemical properties A) B) C) D) Include changes of state of a substance Include mass and color Include changes that alter the identity of a substance Can be observed without altering the identity of a substance

Please make your selection �The sum of the protons and neutrons in an atom equals the ____. �A atomic mass �B mass number �C atomic number �D nucleus number

Please make your selection The study of substances containing carbon is A) Organic chemistry B) Inorganic chemistry C) Analytical chemistry D) Biochemistry

Please make your selection �In a chemical reaction, the type of products obtained is largely determined by which part of the reacting chemicals? A protons B neutrons C nuclei D electrons

Please make your selection �Which of the following elements has the smallest first ionization energy? �A potassium �B magnesium �C sodium �D calcium

Please make your selection �The modern periodic table is arranged in order of increasing atomic ____. �A radius �B mass �C number �D charge

Please make your selection �Which of the following is a chemical property? �A ability to react with oxygen �B color �C freezing point �D hardness

Please make your selection �The first letter in a properly written chemical symbol is always �A underlined. �B capitalized. �C italicized. �D bold faced.

Please make your selection �Which of the following changes to a metal is a chemical change? �A melting �B polishing �C bending �D rusting

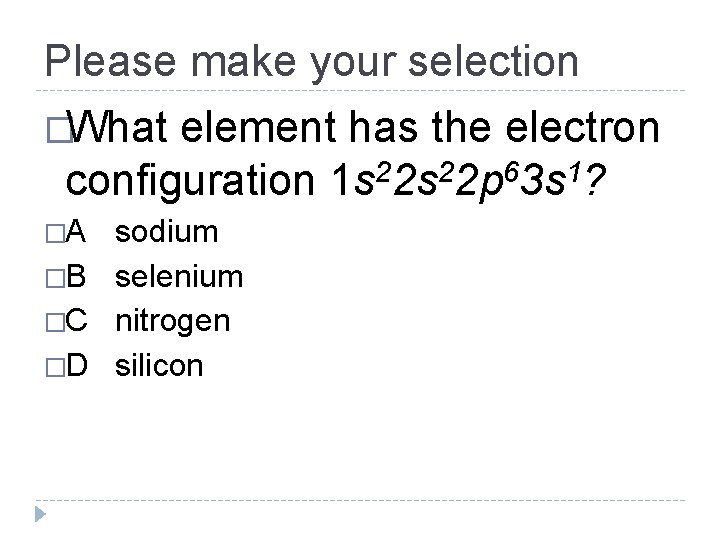
Please make your selection �What element has the electron configuration 1 s 22 p 63 s 1? �A sodium �B selenium �C nitrogen �D silicon

Please make your selection �The letter "p" in the symbol 4 p indicates the ____. �A orbital shape �B principle energy level �C speed of an electron �D spin of an electron

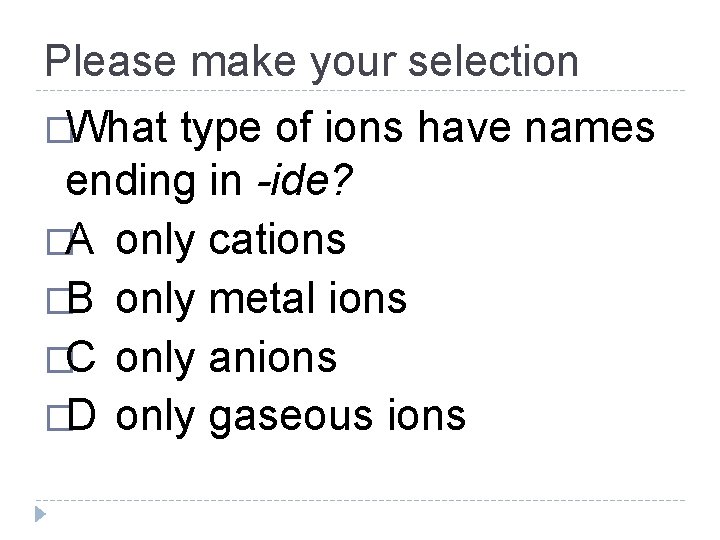
Please make your selection �What type of ions have names ending in -ide? �A only cations �B only metal ions �C only anions �D only gaseous ions

Please make your selection �Who was the man who lived from 460 B. C. – 370 B. C. and was among the first to suggest the idea of atoms? �A Atomos �B Democritus �C Thomson �D Dalton

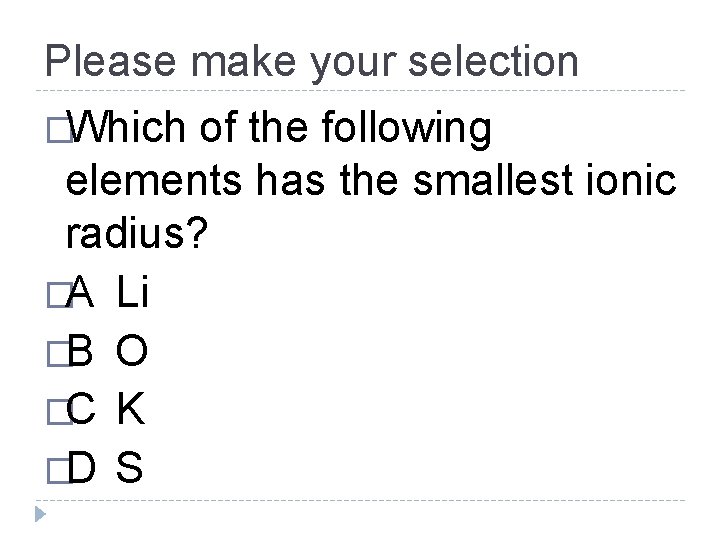
Please make your selection �Which of the following elements has the smallest ionic radius? �A Li �B O �C K �D S

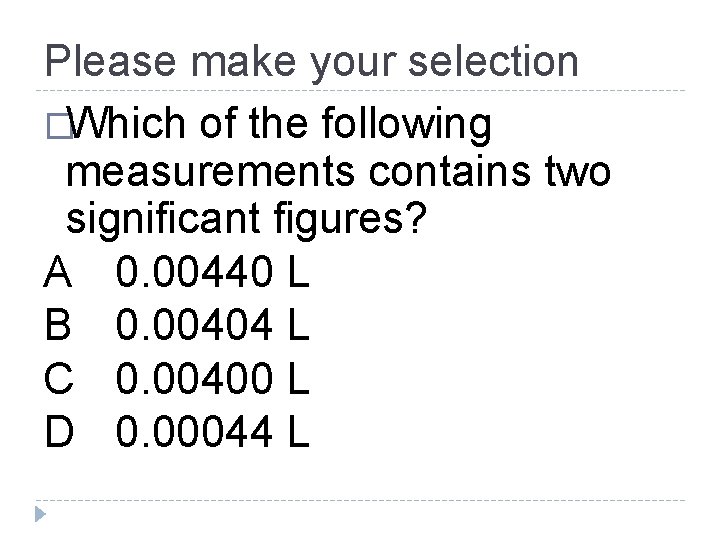
Please make your selection �Which of the following measurements contains two significant figures? A 0. 00440 L B 0. 00404 L C 0. 00400 L D 0. 00044 L

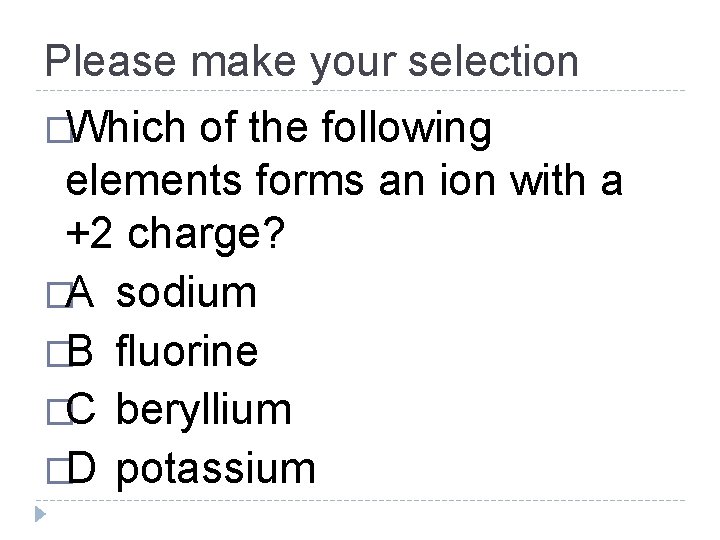
Please make your selection �Which of the following elements forms an ion with a +2 charge? �A sodium �B fluorine �C beryllium �D potassium

Please make your selection �Which state of matter has a definite volume and takes the shape of its container? �A solid �B liquid only �C gas only �D both b and c

Please make your selection �What is the charge on the fluoride anion? �A 2+ + �B 1 �C 1– �D 2–

Please make your selection �What is the element with the highest electronegativity value? �A fluorine �B calcium �C cesium �D helium

Please make your selection �What is the element with the lowest electronegativity value? �A fluorine �B calcium �C cesium �D helium

Please make your selection �How many significant figures are in the measurement 800. 00 grams? �A two �B five �C four �D three

Please make your selection �Isotopes of the same element have different A chemical behavior. B positions on the periodic table. C mass numbers. D atomic numbers.

Please make your selection �Express the sum of 7. 68 m and 5. 0 m using the correct number of significant digits. A 13 m B 12. 68 m C 12. 7 m D 10 m

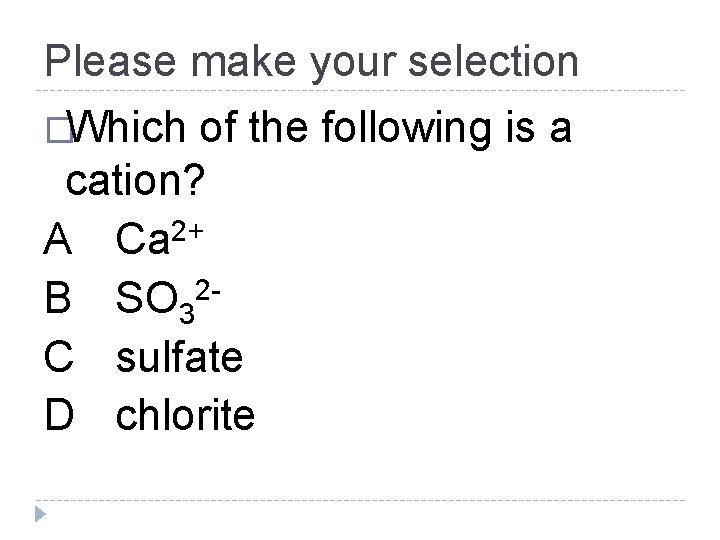
Please make your selection �Which of the following is a cation? A Ca 2+ 2 B SO 3 C sulfate D chlorite

Please make your selection �Which state of matter takes both the shape and volume of its container? A solid B liquid only C gas only D both b and c

Please make your selection How many electrons can occupy the s orbital at each energy level? a. b. c. d. Two, if they have opposite spins Two, if they have the same spin One No more than eight

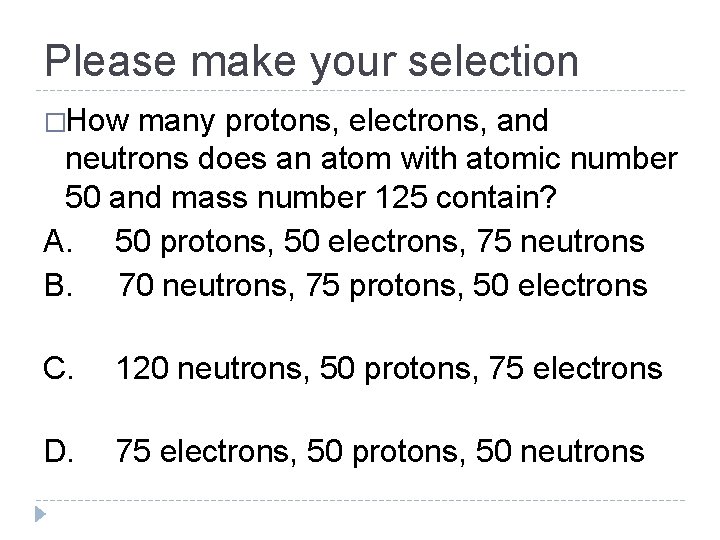
Please make your selection �How many protons, electrons, and neutrons does an atom with atomic number 50 and mass number 125 contain? A. 50 protons, 50 electrons, 75 neutrons B. 70 neutrons, 75 protons, 50 electrons C. 120 neutrons, 50 protons, 75 electrons D. 75 electrons, 50 protons, 50 neutrons

Please make your selection Divide 5. 7 m by 2 m. The quotient is correctly reported as a. b. c. d. 2. 8 m 2. 85 m 2. 9 m 3 m

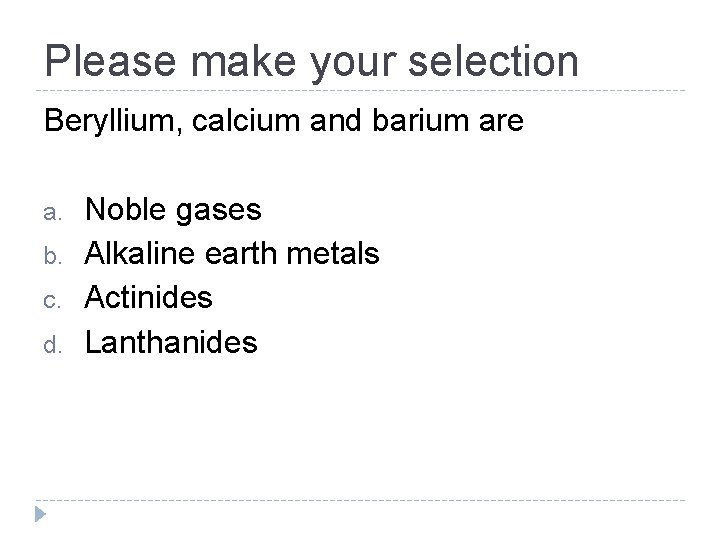
Please make your selection Beryllium, calcium and barium are a. b. c. d. Noble gases Alkaline earth metals Actinides Lanthanides

Please make your selection Mendeleev is credited with developing the first successful a. b. c. d. Periodic table Method of determining atomic number Test for radioactivity Use of x-rays

Which of the following shows a physical change occurring? a. peach spoils. b. copper bowl tarnishes. c. The copper bracelet turns your skin green. d. The hot-glue gun melts a glue stick.

Please make your selection Which element listed is considered to be unreactive (inert) based on its location on the periodic table? a. b. c. d. H He S F

Please make your selection A neutral atom of selenium contains a. b. c. d. 34 electrons 78. 96 electrons 45 electrons 79 electrons

Which of the following observations does not indicate that a chemical change has occurred? �a. change of state �b. formation of a precipitate �c. absorption of energy �d. release of a gas

Please make your selection The most useful source of chemical information about the elements is a a. Calculator b. Table of metric equivalents c. Periodic table d. Table of isotopes

Please make your selection A vertical column of blocks in the periodic table is called a(n) a. b. c. d. Period Group Property Octet

Please make your selection The elements that border the zigzag line in the periodic table are a. b. c. d. Inactive Metals Metalloids nonmetals

Please make your selection Which of the following is quantitative? a. b. c. d. The liquid turns blue litmus paper red. The liquid boils at 100 o. C. The liquid tastes bitter. The liquid is cloudy.

Please make your selection Quantitative observations are recorded using a. b. c. d. Numerical information A control Non-numerical information A system

Please make your selection The symbol for the metric unit used to measure mass is a. b. c. d. m mm g L

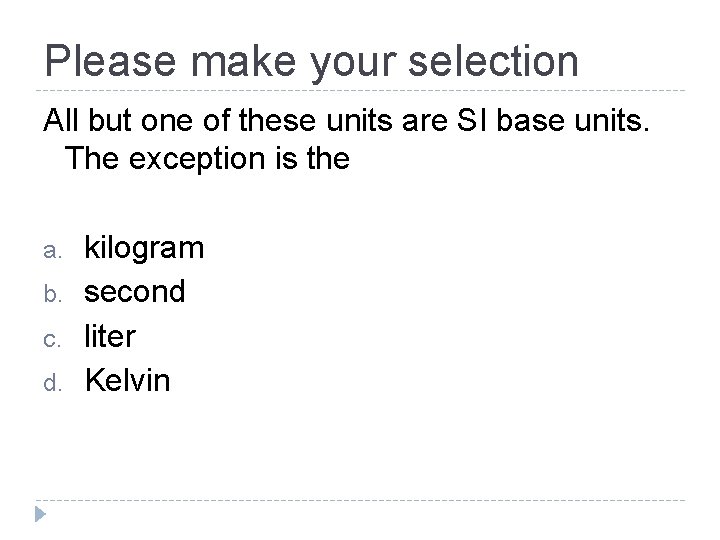
Please make your selection All but one of these units are SI base units. The exception is the a. b. c. d. kilogram second liter Kelvin

Please make your selection Elements in a group in the periodic table can be expected to have similar a. Atomic masses b. Atomic numbers c. Number of neutrons d. properties

Please make your selection Barium, atomic number 56, is the fifth element in Group 2. What is the atomic number of radium, the next element in group 2? a. 64 b. 74 c. 88 d. 103

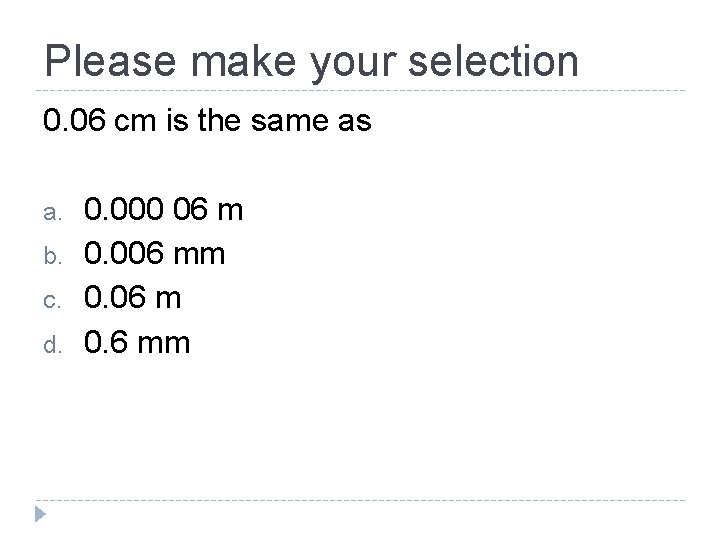
Please make your selection 0. 06 cm is the same as a. b. c. d. 0. 000 06 m 0. 006 mm 0. 06 m 0. 6 mm

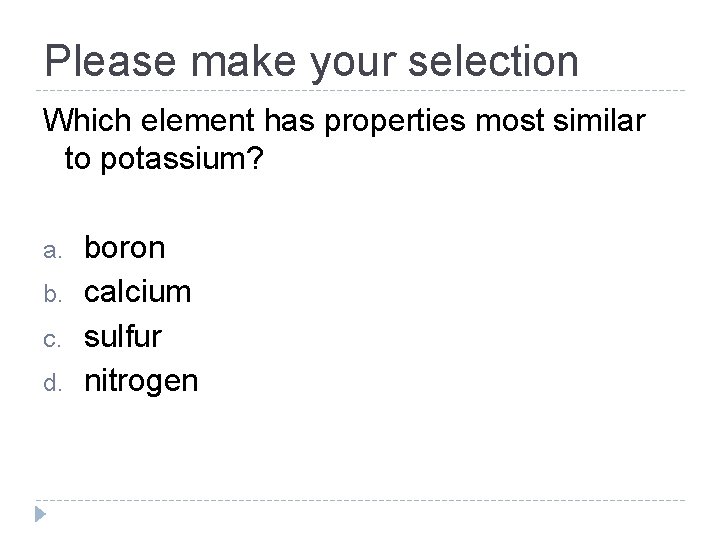
Please make your selection Which element has properties most similar to potassium? a. b. c. d. boron calcium sulfur nitrogen

Please make your selection Which of the following is qualitative? a. b. c. d. A chemical reaction is complete in 2. 3 seconds. The solid has a mass of 23. 4 grams. The p. H of a liquid is 5. Salt deposits form from an evaporated liquid.

Please make your selection The density of aluminum is 2. 70 g/cm 3. The volume of a solid piece of aluminum is 1. 50 cm 3. Find its mass. a. b. c. d. 1. 50 g 2. 70 g 1. 80 g 4. 05 g

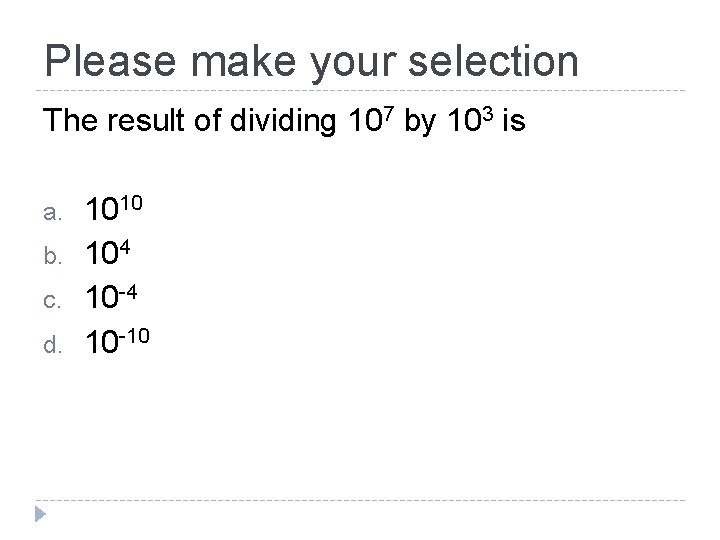
Please make your selection The result of dividing 107 by 103 is a. b. c. d. 1010 104 10 -10

Please make your selection The particles in a solid are a. b. c. d. packed closely together constantly in motion very far apart able to slide past each other

Please make your selection A chemist who frequently carries out a complex experiment is likely to have high a. b. c. d. Accuracy, but low precision Precision, but low accuracy Accuracy Precision

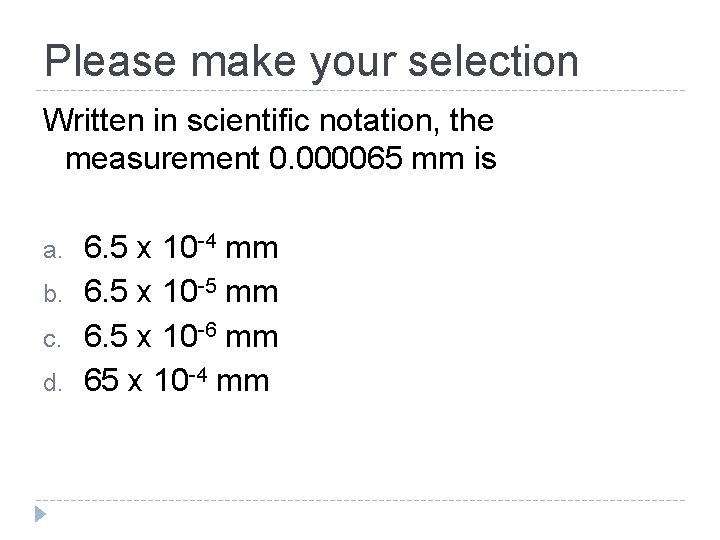
Please make your selection Written in scientific notation, the measurement 0. 000065 mm is a. b. c. d. 6. 5 x 10 -4 mm 6. 5 x 10 -5 mm 6. 5 x 10 -6 mm 65 x 10 -4 mm

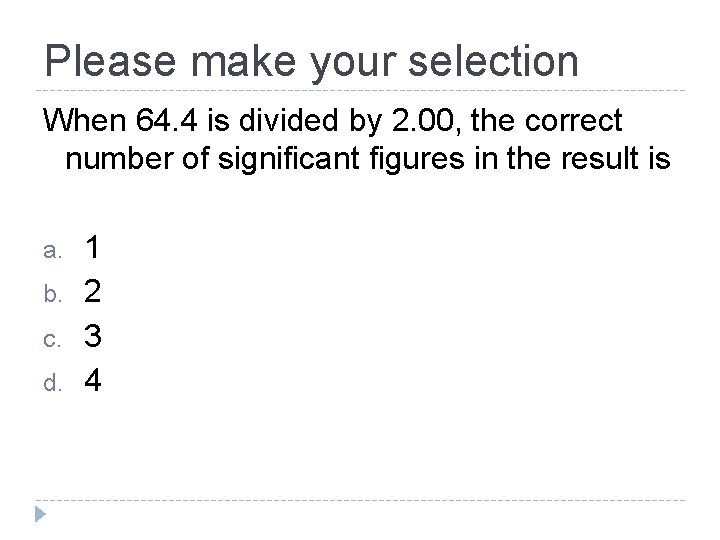
Please make your selection When 64. 4 is divided by 2. 00, the correct number of significant figures in the result is a. b. c. d. 1 2 3 4

Please make your selection Visible light, X rays, infrared radiation, and radio waves all have the same a. b. c. d. Energy Wavelength Energy Speed