Unit 2 Matter III Properties Changes in Matter










- Slides: 12

Unit 2 - Matter III. Properties & Changes in Matter (p. 11 -14) w Extensive vs. Intensive w Physical vs. Chemical C. Johannesson

A. Extensive vs. Intensive ö Extensive Property w depends on the amount of matter present ö Intensive Property w depends on the identity of substance, not the amount C. Johannesson

A. Extensive vs. Intensive ö Examples: w boiling point intensive w volume extensive w mass extensive w density intensive w conductivity intensive C. Johannesson

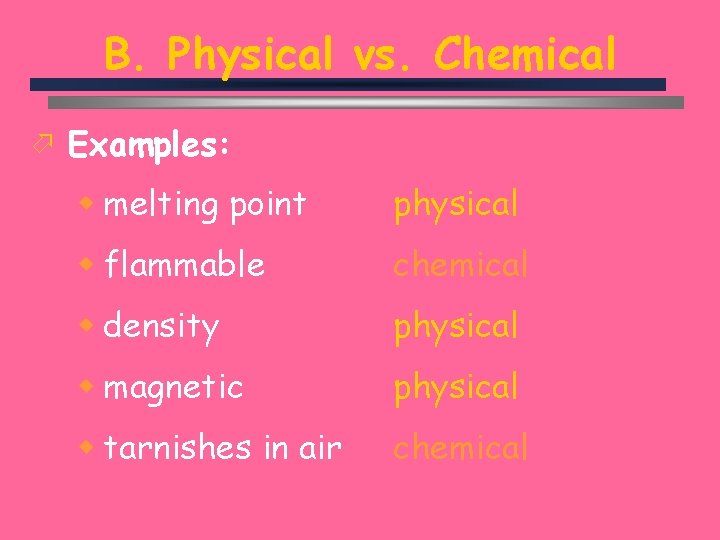
B. Physical vs. Chemical ö Physical Property w can be observed without changing the identity of the substance ö Chemical Property w describes the ability of a substance to undergo changes in identity C. Johannesson

B. Physical vs. Chemical ö Examples: w melting point physical w flammable chemical w density physical w magnetic physical w tarnishes in air chemical C. Johannesson

B. Physical vs. Chemical ö Physical Change w changes the form of a substance without changing its identity w properties remain the same ö Chemical Change w changes the identity of a substance w products have. C. Johannesson different properties

Physical Changes in Matter ö change in a substance that doesn’t change the identity of the substance ö Ex. grinding, cutting, melting, boiling ö Can be reversible, or irreversible ö Includes all changes of state (physical changes of a substance from one state to another)

Chemical Changes in Matter ö a change in which a substance is ö ö converted into a different substance same as chemical reaction doesn’t change the amount of matter present reactants- substances that react products- substances that form ö Arrow points from the reactants to the new products

B. Physical vs. Chemical ö Signs of a Chemical Change (Reaction) w change in color or odor w formation of a gas (bubbling or fizzing) w formation of a precipitate (solid) w change in light or heat C. Johannesson

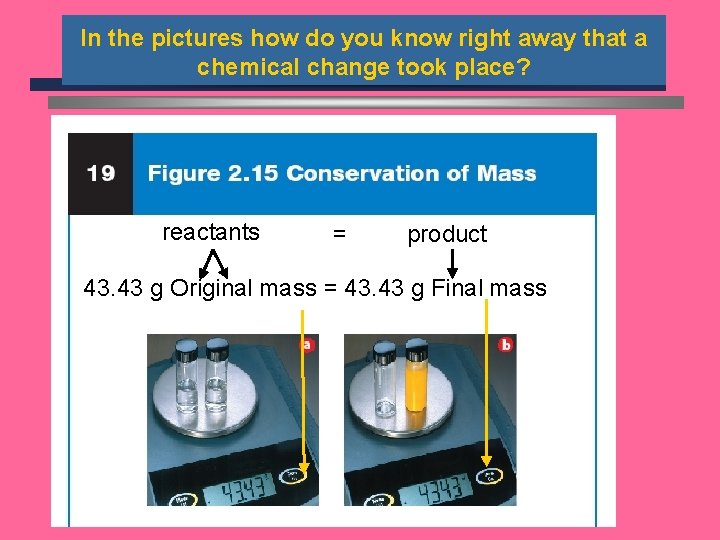
Conservation of Mass ö During any chemical reaction, the mass of the products is always equal to the mass of the reactants. ö All the mass can be accounted for: w Burning of wood results in products that appear to have less mass as ashes; where is the rest? ö Law of conservation of mass

In the pictures how do you know right away that a chemical change took place? reactants = product 43. 43 g Original mass = 43. 43 g Final mass

B. Physical vs. Chemical ö Examples: w rusting iron chemical w dissolving in water physical w burning a log chemical w melting ice physical w grinding spices physical C. Johannesson