Unit 1 Whats a MATTER Match This Matter





- Slides: 14

Unit #1: What’s a MATTER?

Match This Matter Mass Weight - Does not change based on location Used to measure the amount of “stuff” an object has Changes on location Anything that has “mass” or “stuff” and takes up space The measure of the force of gravity The amount of “stuff” or “Matter” in an object

Matter Anything that has “stuff” and takes up space Mass The amount of “stuff” or “Matter” in an object Does NOT change based on location Weight The measure of the force of gravity Changes based on location – Gravity Changes Used to measure the amount of “stuff” an object has

What’s Your Weight? 30 lbs. 185 lbs. 65 lbs.

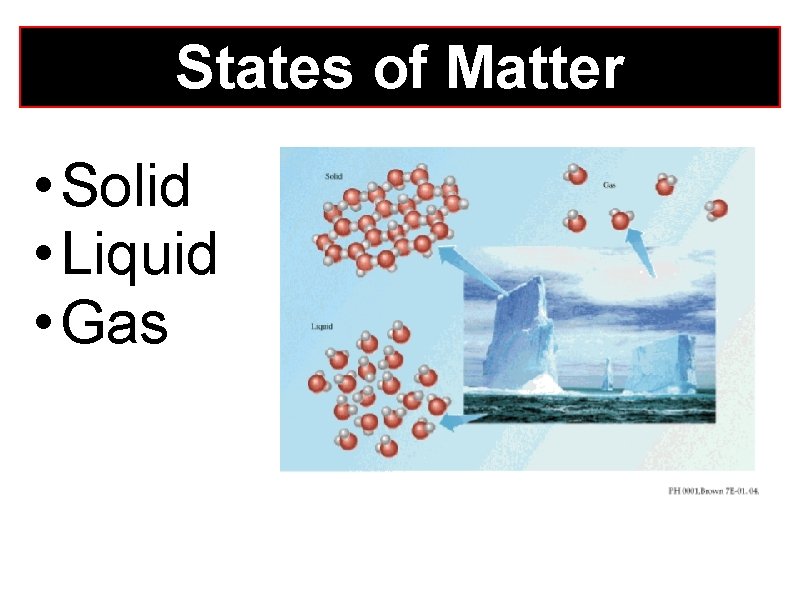
States of Matter • Solid • Liquid • Gas

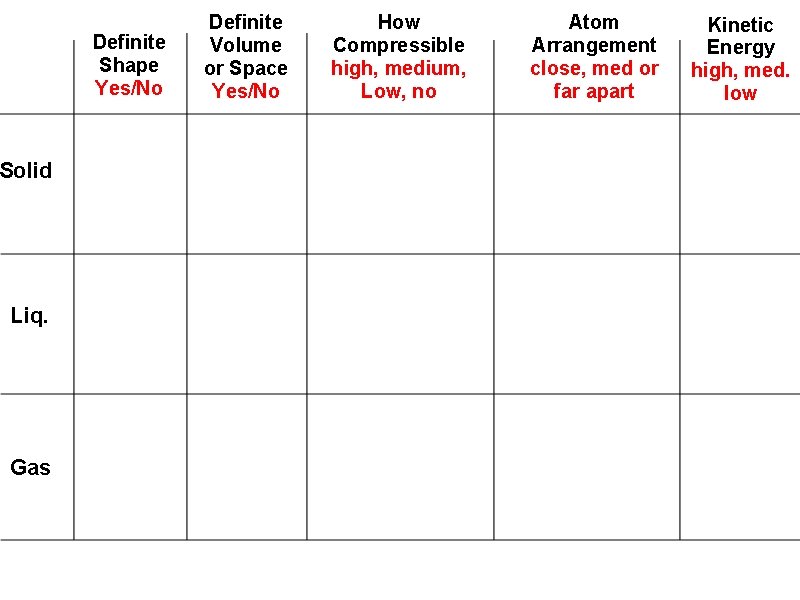
Definite Shape Yes/No Solid Liq. Gas Definite Volume or Space Yes/No How Compressible high, medium, Low, no Atom Arrangement close, med or far apart Kinetic Energy high, med. low

States of Matter Song http: //www. youtube. com/watch? v=V 9 WYwe. BA 6 v. A

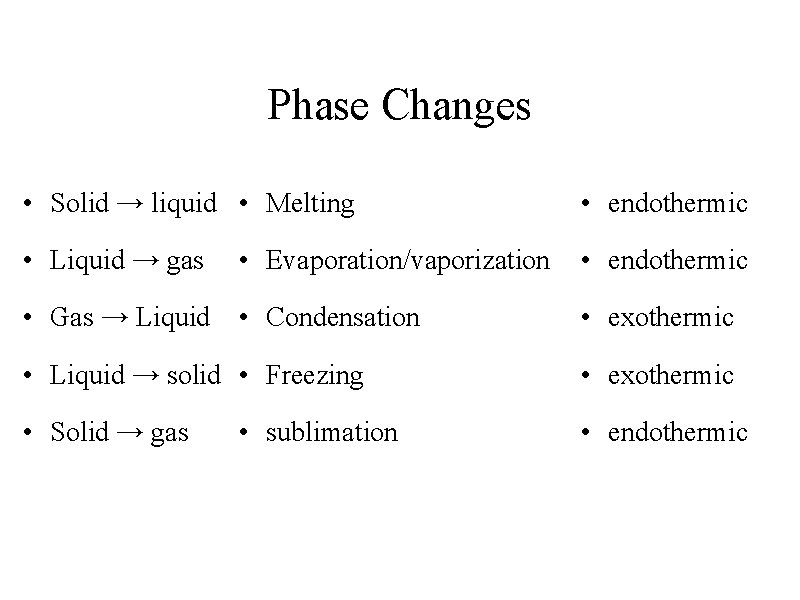
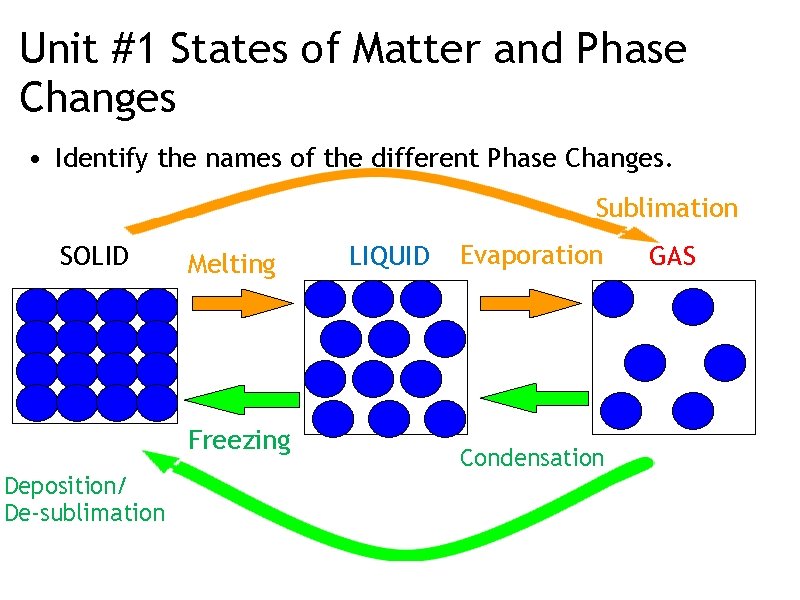
Phase Changes • Solid → liquid • Melting • endothermic • Liquid → gas • Evaporation/vaporization • endothermic • Gas → Liquid • Condensation • exothermic • Liquid → solid • Freezing • exothermic • Solid → gas • endothermic • sublimation

Unit #1: Introduction to Matter States of Matter and Phase Changes • Draw Three (3) Boxes equally spaced across your paper like so:

Unit #1: Introduction to Matter States of Matter and Phase Changes • Label the Left Box as "Solid", the Middle Box as "Liquid", and the Right Box as "Gas" SOLID LIQUID GAS

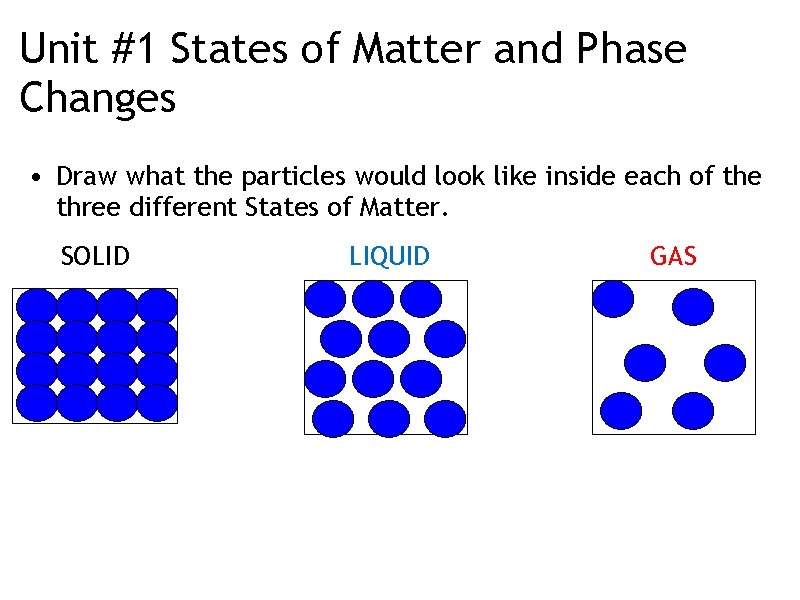
Unit #1 States of Matter and Phase Changes • Draw what the particles would look like inside each of the three different States of Matter. SOLID LIQUID GAS

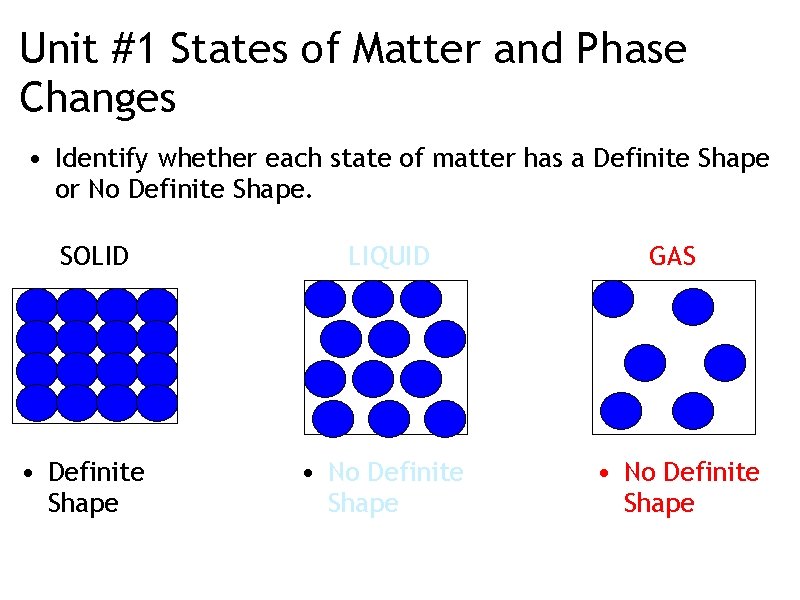
Unit #1 States of Matter and Phase Changes • Identify whether each state of matter has a Definite Shape or No Definite Shape. SOLID • Definite Shape LIQUID GAS • No Definite Shape

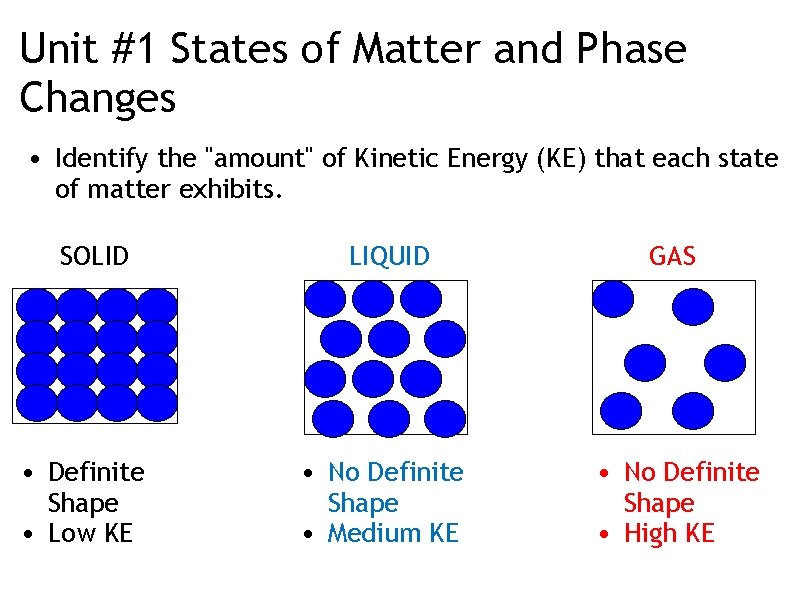
Unit #1 States of Matter and Phase Changes • Identify the "amount" of Kinetic Energy (KE) that each state of matter exhibits. SOLID • Definite Shape • Low KE LIQUID GAS • No Definite Shape • Medium KE • No Definite Shape • High KE

Unit #1 States of Matter and Phase Changes • Identify the names of the different Phase Changes. Sublimation SOLID Melting Freezing Deposition/ De-sublimation LIQUID Evaporation Condensation GAS