UCLA Lung Cancer Chemoprevention Trials Jenny T Mao







- Slides: 27

UCLA Lung Cancer Chemoprevention Trials Jenny T. Mao, M. D. , FCCP Associate Professor Division of Pulmonary and Critical Care David Geffen School of Medicine at UCLA

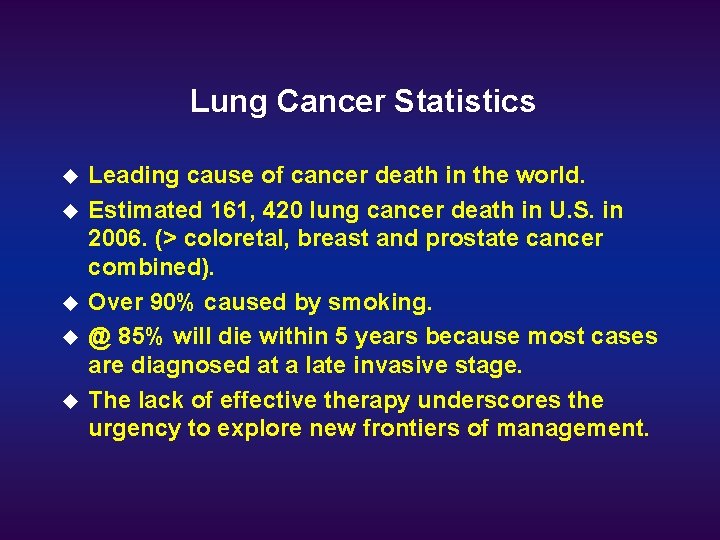
Lung Cancer Statistics u u u Leading cause of cancer death in the world. Estimated 161, 420 lung cancer death in U. S. in 2006. (> coloretal, breast and prostate cancer combined). Over 90% caused by smoking. @ 85% will die within 5 years because most cases are diagnosed at a late invasive stage. The lack of effective therapy underscores the urgency to explore new frontiers of management.

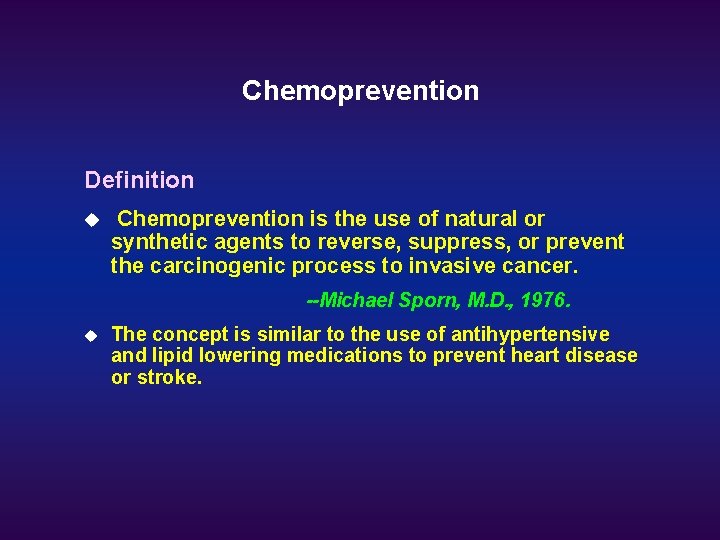
Chemoprevention Definition u Chemoprevention is the use of natural or synthetic agents to reverse, suppress, or prevent the carcinogenic process to invasive cancer. --Michael Sporn, M. D. , 1976. u The concept is similar to the use of antihypertensive and lipid lowering medications to prevent heart disease or stroke.

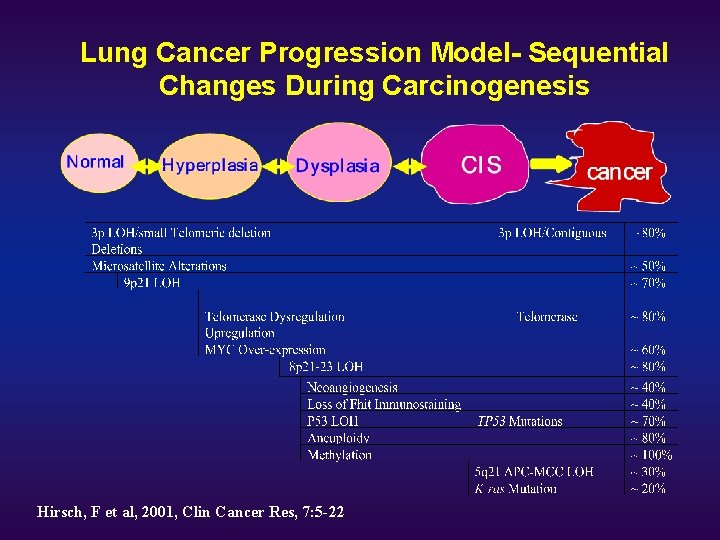
Lung Cancer Progression Model- Sequential Changes During Carcinogenesis Hirsch, F et al, 2001, Clin Cancer Res, 7: 5 -22

Goals of Chemoprevention u At the cellular level, inhibit the mechanisms that may lead to or facilitate malignant transformation. u At the tissue level, reverse and/or prevent the development or progression of premalignant lesions. u At the clinical level, reduce the incidence of cancer.

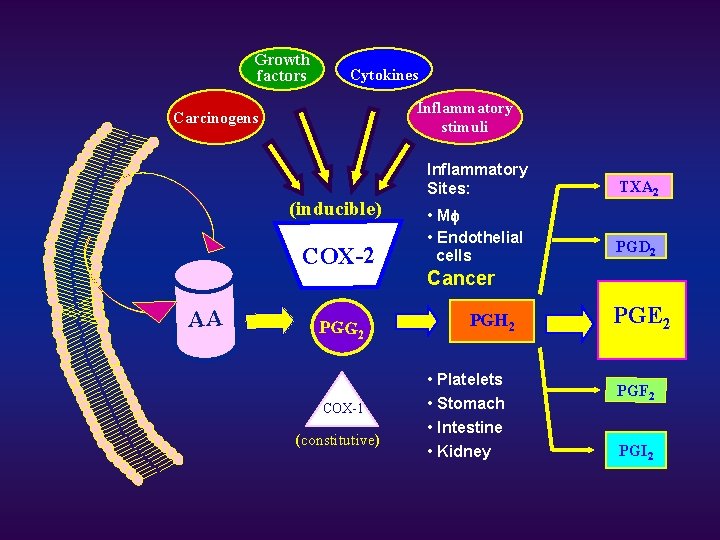
Growth factors Cytokines Inflammatory stimuli Carcinogens (inducible) COX-2 AA PGG 2 COX-1 (constitutive) Inflammatory Sites: TXA 2 • Mf • Endothelial cells PGD 2 Cancer PGH 2 • Platelets • Stomach • Intestine • Kidney PGE 2 PGF 2 PGI 2

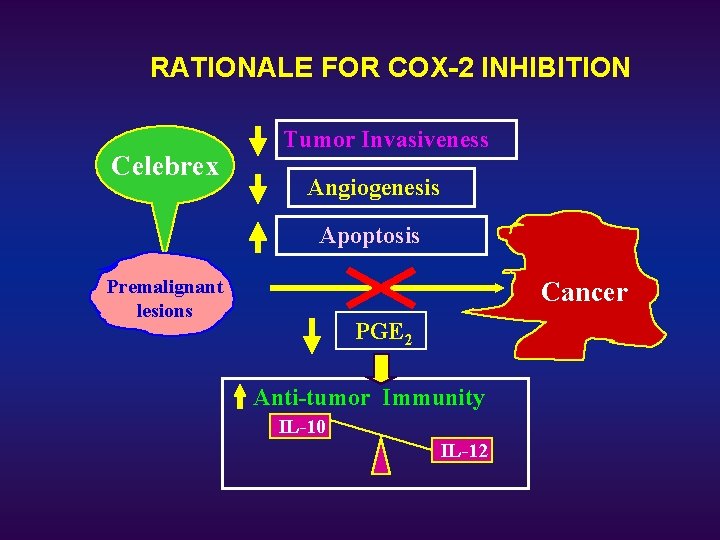
RATIONALE FOR COX-2 INHIBITION Celebrex Tumor Invasiveness Angiogenesis Apoptosis Premalignant lesions Cancer PGE 2 Anti-tumor Immunity IL-10 IL-12

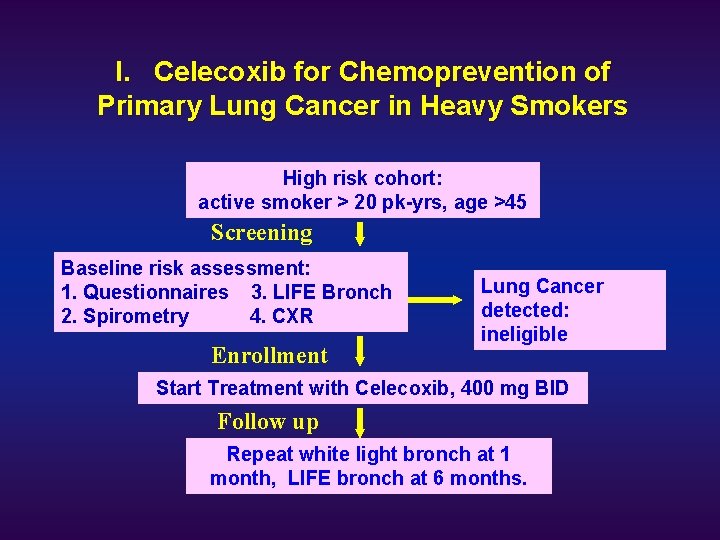
I. Celecoxib for Chemoprevention of Primary Lung Cancer in Heavy Smokers High risk cohort: active smoker > 20 pk-yrs, age >45 Screening Baseline risk assessment: 1. Questionnaires 3. LIFE Bronch 2. Spirometry 4. CXR Lung cancer detected Enrollment Lung Cancer detected: ineligible Start Treatment with Celecoxib, 400 mg BID Follow up Repeat white light bronch at 1 month, LIFE bronch at 6 months.

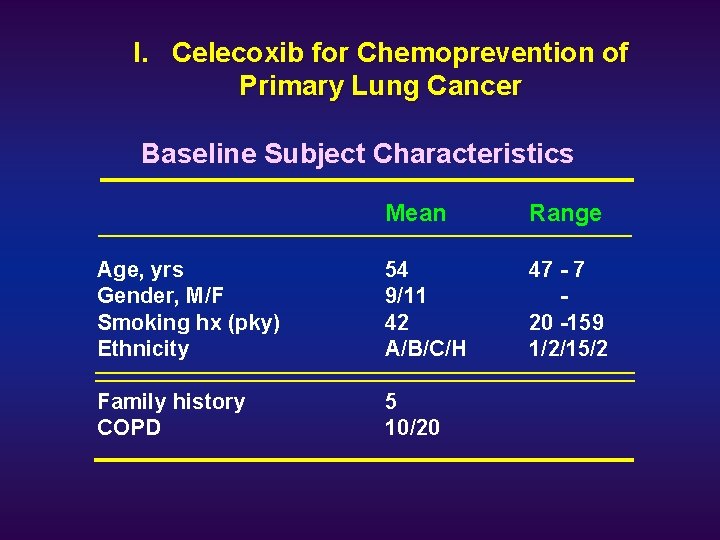
I. Celecoxib for Chemoprevention of Primary Lung Cancer Baseline Subject Characteristics Mean Range Age, yrs Gender, M/F Smoking hx (pky) Ethnicity 54 9/11 42 A/B/C/H 47 - 7 20 -159 1/2/15/2 Family history COPD 5 10/20

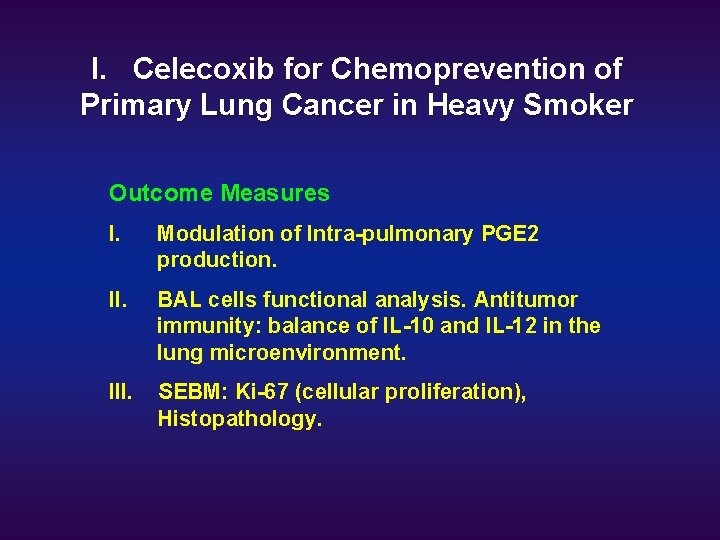
I. Celecoxib for Chemoprevention of Primary Lung Cancer in Heavy Smoker Outcome Measures I. Modulation of Intra-pulmonary PGE 2 production. II. BAL cells functional analysis. Antitumor immunity: balance of IL-10 and IL-12 in the lung microenvironment. III. SEBM: Ki-67 (cellular proliferation), Histopathology.

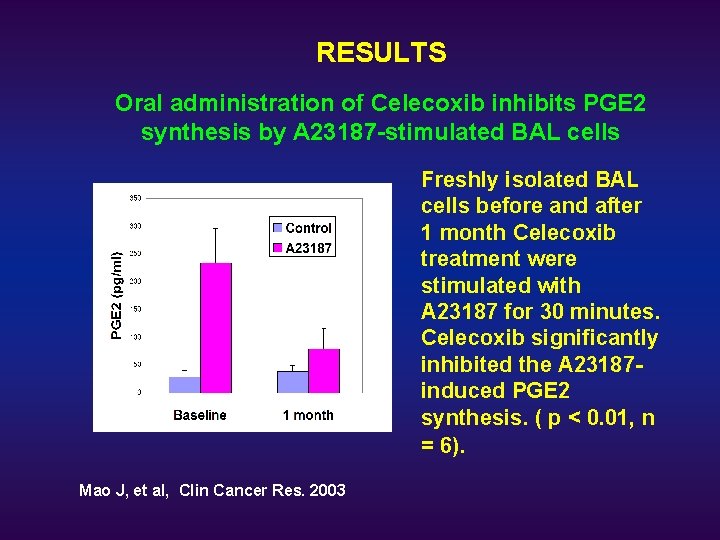
RESULTS Oral administration of Celecoxib inhibits PGE 2 synthesis by A 23187 -stimulated BAL cells Freshly isolated BAL cells before and after 1 month Celecoxib treatment were stimulated with A 23187 for 30 minutes. Celecoxib significantly inhibited the A 23187 induced PGE 2 synthesis. ( p < 0. 01, n = 6). Mao J, et al, Clin Cancer Res. 2003

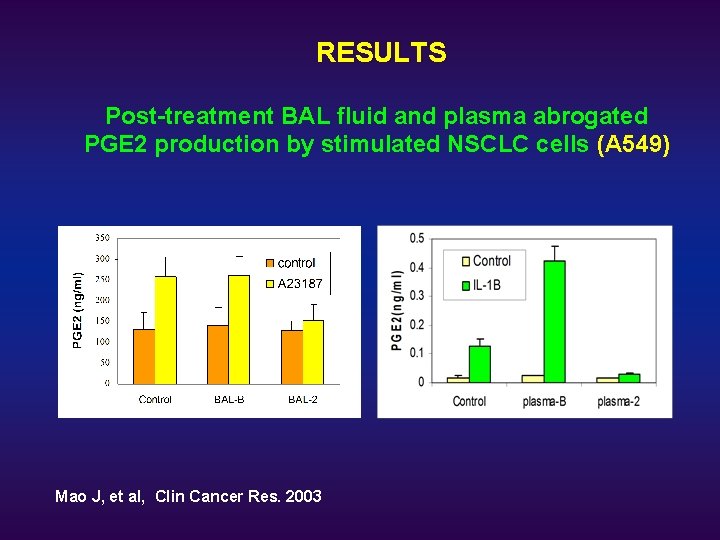
RESULTS Post-treatment BAL fluid and plasma abrogated PGE 2 production by stimulated NSCLC cells (A 549) Mao J, et al, Clin Cancer Res. 2003

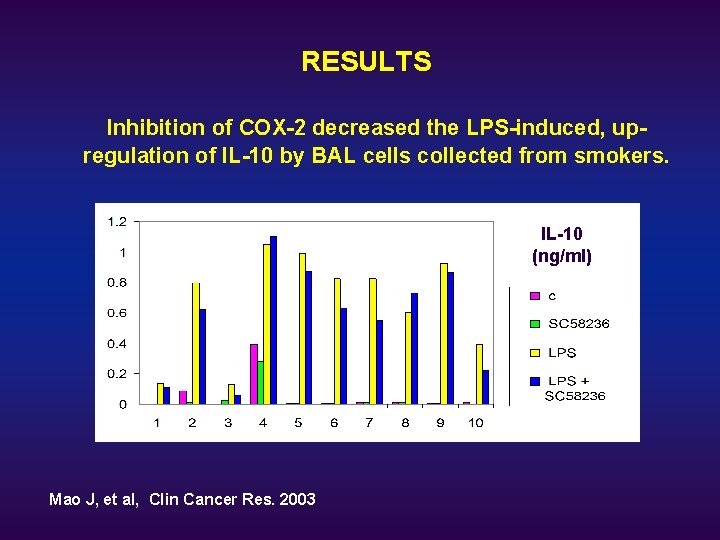
RESULTS Inhibition of COX-2 decreased the LPS-induced, upregulation of IL-10 by BAL cells collected from smokers. IL-10 (ng/ml) Mao J, et al, Clin Cancer Res. 2003

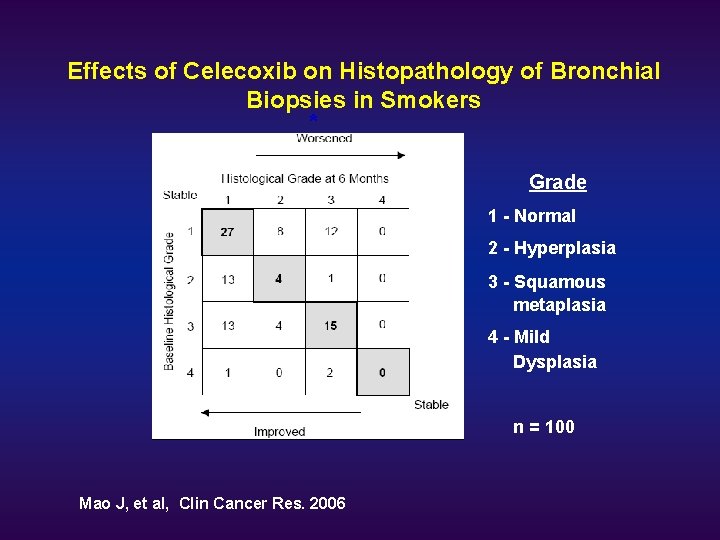
Effects of Celecoxib on Histopathology of Bronchial Biopsies in Smokers * Grade 1 - Normal 2 - Hyperplasia 3 - Squamous metaplasia 4 - Mild Dysplasia n = 100 Mao J, et al, Clin Cancer Res. 2006

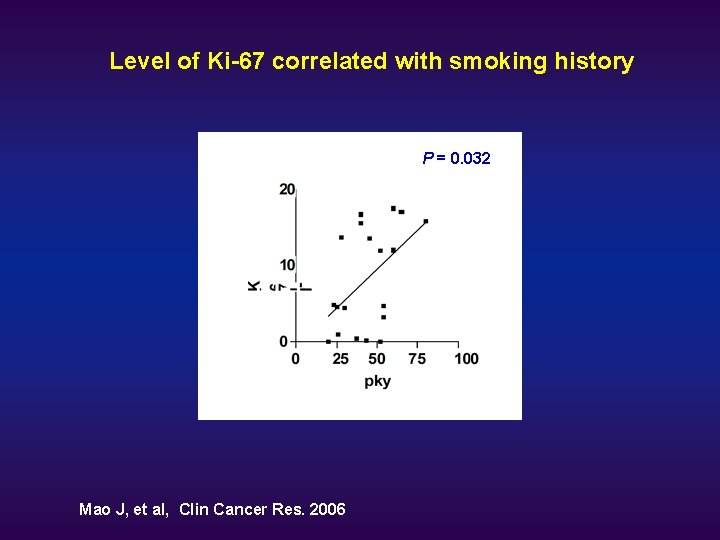
Ki-67 u u u Ki-67 is a proliferation marker expressed in all phases of the cell cycle except in resting cells. Abnormal epithelial proliferation is a hallmark of tumorigenesis. Elevated Ki-67 expression is associated with poor prognosis. Elevated Ki-67 levels can be detected in areas where squamous metaplasia is lacking. Ki-67 may be a useful marker for lung cancer risk.

Level of Ki-67 correlated with smoking history A * Mao J, et al, Clin Cancer Res. 2006 P = 0. 032

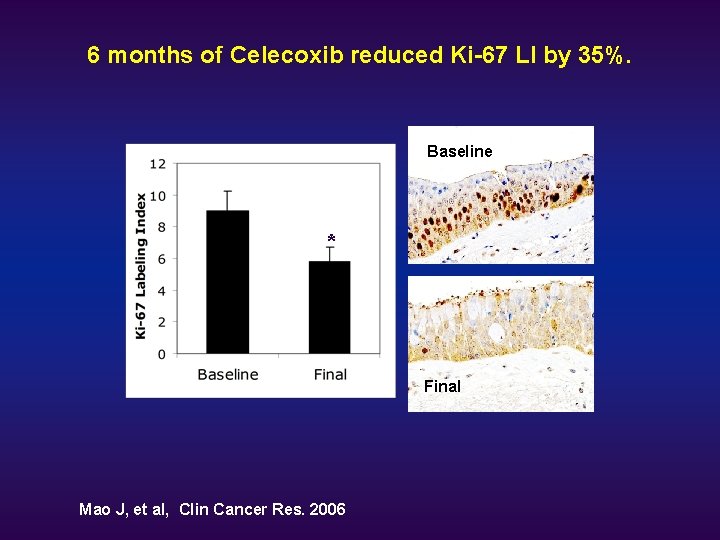
6 months of Celecoxib reduced Ki-67 LI by 35%. Baseline * Final Mao J, et al, Clin Cancer Res. 2006

Summary from the Phase IIa Smokers Study u Oral Celecoxib blocked the capacity of PGE 2 production by smokers’ AM. u Plasma and BAL fluid obtained from treated subjects blocked PGE 2 production by stimulated NSCLC cells A 549 in vitro. u Inhibition of COX-2 blocked the release of IL-10 by LPS stimulated AM from smokers, may restore anti-tumor immunity. u Oral Celecoxib decreased Ki-67 LI in bronchial biopsies, indicating that celecoxib may be capable of favorably modulating the proliferation indices in bronchial tissue of active smokers. u These findings support the continued investigation of COX-2 inhibitor in lung cancer chemoprevention.

II. Lung Cancer Chemoprevention with Celecoxib in Ex-Smokers Overall Objectives u To determine the feasibility of Celecoxib for chemoprevention of lung cancer in high risk exsmokers. Celecoxib is being evaluated for its impact on cellular and molecular events associated with lung carcinogenesis: 1) modulation of a panel of biomarkers of field cancerization, 2) regulation of arachidonic acid metabolism, 3) antitumor immunity 4) angiogenesis in the lung microenvironment.

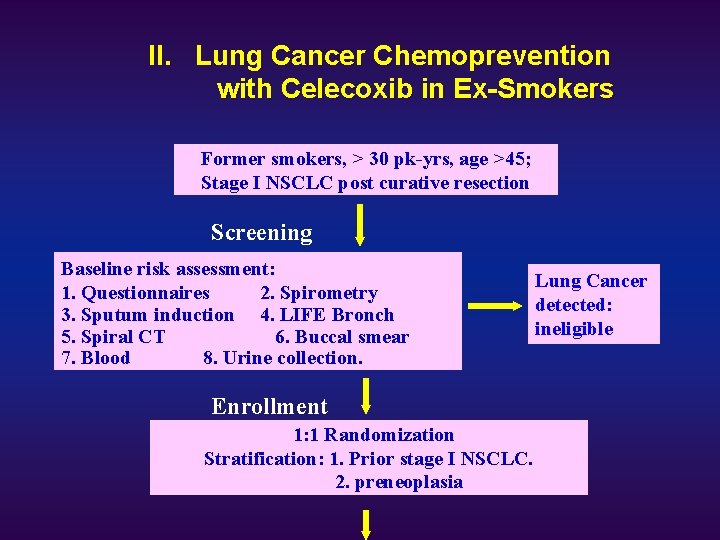
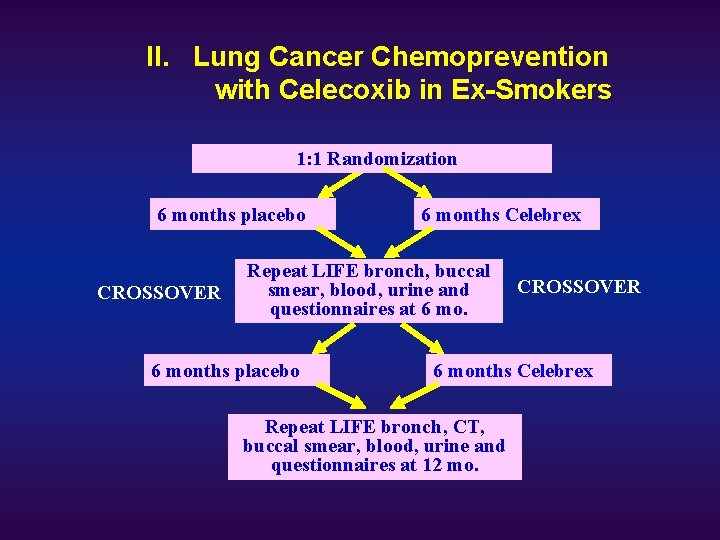
II. Lung Cancer Chemoprevention with Celecoxib in Ex-Smokers Former smokers, > 30 pk-yrs, age >45; Stage I NSCLC post curative resection Screening Baseline risk assessment: 1. Questionnaires 2. Spirometry 3. Sputum induction 4. LIFE Bronch 5. Spiral CT 6. Buccal smear 7. Blood 8. Urine collection. Enrollment 1: 1 Randomization Stratification: 1. Prior stage I NSCLC. Follow up 2. preneoplasia Lung Cancer detected: ineligible

II. Lung Cancer Chemoprevention with Celecoxib in Ex-Smokers 1: 1 Randomization 6 months placebo CROSSOVER 6 months Celebrex Repeat LIFE bronch, buccal smear, blood, urine and questionnaires at 6 months placebo CROSSOVER 6 months Celebrex Repeat LIFE bronch, CT, buccal smear, blood, urine and questionnaires at 12 mo.

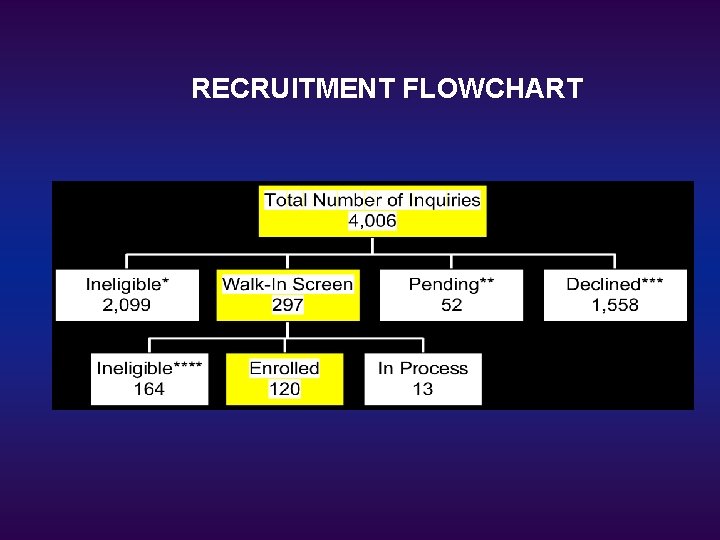
RECRUITMENT FLOWCHART

Laboratory Studies and SEBM 1. Bronchial biospsies Immunostaining: Ki-67, COX-2, EGFR, p 16, p 27, cyclin D 1 & E, bcl-2, p 53, CD 44, Histopathology Frozen biopsy: CXC chemokines. RNA from homogenates. DNA analysis : GSTP 1, p 16 methylation. GSTP 1, GSTM 1, CYP 1 A 1, P 53 polymorphisims. 3. Sputum: Cytology Immunostaining 6. Urine 2. BAL Fluid: PGE 2, IL 10, IL-12, VGEF, CXC chemokines, MMP, TIMP-1, LTB 4 Cytology Alveolar Macrophages: 1. Functional analysis. 2. RNA 7. Primary Tumor: 4. Blood Plasma: PGE 2 Buffy coat 5. Buccal Smear DNA analysis Cox-2

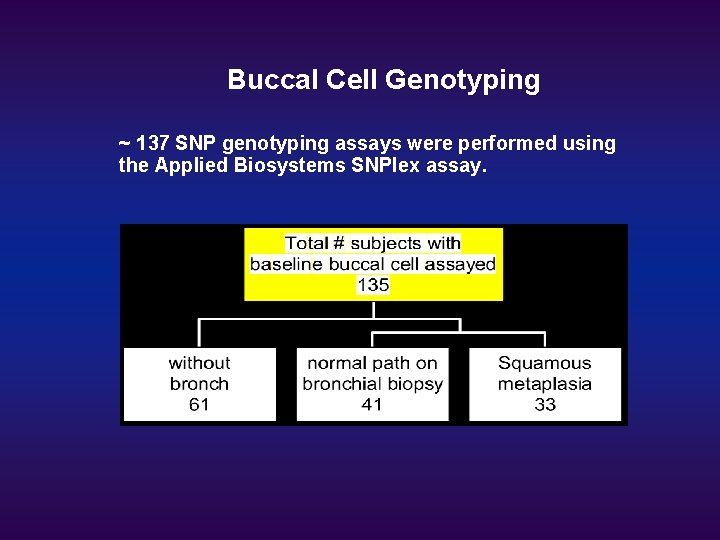
Buccal Cell Genotyping ~ 137 SNP genotyping assays were performed using the Applied Biosystems SNPlex assay.

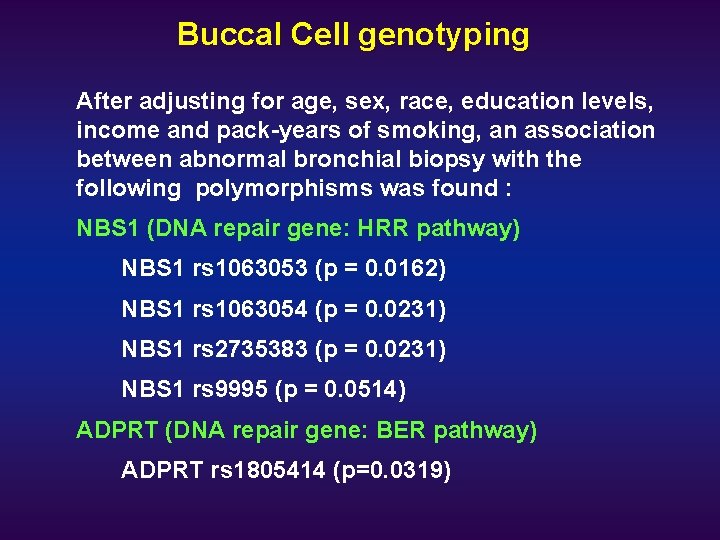
Buccal Cell genotyping After adjusting for age, sex, race, education levels, income and pack-years of smoking, an association between abnormal bronchial biopsy with the following polymorphisms was found : NBS 1 (DNA repair gene: HRR pathway) NBS 1 rs 1063053 (p = 0. 0162) NBS 1 rs 1063054 (p = 0. 0231) NBS 1 rs 2735383 (p = 0. 0231) NBS 1 rs 9995 (p = 0. 0514) ADPRT (DNA repair gene: BER pathway) ADPRT rs 1805414 (p=0. 0319)

Acknowledgement Division of Pulmonary & Critical Care S. Dubinett M. Roth R. Strieter D. Tashkin B. Adams J. Bailow F. Baratelli M. Burdick J. Dermand T. Ho M. Hoang V. Nguyen D. Ritter A. Tsu L. Ying L. Zhu Thoracic Surgery E. C. Holmes R. Cameron S. Perez Thoracic Imaging D. Aberle Pathology M. Fishbein J. Y. Rao Oncology R. Figlin Biostatistics Epidemiology R. Elashoff H-J. Wang Z-F. Zhang UCSD K. Serio C Chun W. Cao

SUPPORT u u u u TRDRP CRFA NCI/K 23 NCI/UO 1 UCLA Lung Cancer SPORE Stop Cancer Miller Family Grant Study drugs from Pfizer, Inc.
 Jenny mao
Jenny mao Mao mao
Mao mao Optimal lung cancer pathway
Optimal lung cancer pathway Tnm stage lung cancer
Tnm stage lung cancer Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool Lifetime risk of lung cancer
Lifetime risk of lung cancer Di vs siadh vs csw
Di vs siadh vs csw Short term goal ncp
Short term goal ncp Gesundheit central european lung cancer patient network
Gesundheit central european lung cancer patient network Dr. amy ford
Dr. amy ford Creative commons
Creative commons Random control trials
Random control trials Virtual field trip salem witch trials
Virtual field trip salem witch trials Do we our life done
Do we our life done What caused the salem witch trial hysteria of 1692 dbq
What caused the salem witch trial hysteria of 1692 dbq Ndsu corn variety trials
Ndsu corn variety trials York trials unit
York trials unit Nuremberg trials
Nuremberg trials Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Nida clinical trials network
Nida clinical trials network Ccea agreement trials
Ccea agreement trials Futuresearch trials
Futuresearch trials Clinical trials.gov login
Clinical trials.gov login Dhl bishkek
Dhl bishkek Prs administrator
Prs administrator Clinical research statistician
Clinical research statistician Japanese bridging studies
Japanese bridging studies Repeated bernoulli trials
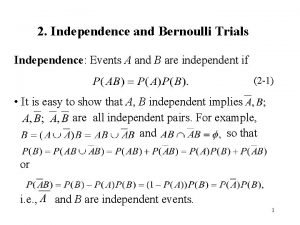
Repeated bernoulli trials