Treatment Of Later Relapse Chemo Resistant Diffuse Large



















- Slides: 30

Treatment Of Later Relapse/ Chemo. Resistant Diffuse Large B Cell Lymphoma FOROUD SHAHBAZI PHARM. D

Introduction 1. Diffuse large B cell lymphoma (DLBCL) is the most common form of aggressive NHL accounting for approximately 30%-40% of cases. 2. The standard frontline treatment is a combination chemo-immunotherapy regimen given for 6 -8 cycles, most commonly R-CHOP (Rituximab, Cyclophosphamide, Adriamycin, and Prednisone) 3. Despite long-term remissions in approximately 60% of patients, for those with high risk features such as single or double hit lymphoma, primary refractory disease, or early relapse within <12 months.

Optimal management for patients who experience two or more relapses of DLBCL is unknown, and selection of treatment depends on prior therapies, available resources, institutional preferences, and patient wishes. For patients with second or later relapse of DLBCL, those who relapse after autologous hematopoietic cell transplantation (HCT), and disease that is chemoresistant to salvage therapy, Participation in a clinical trial

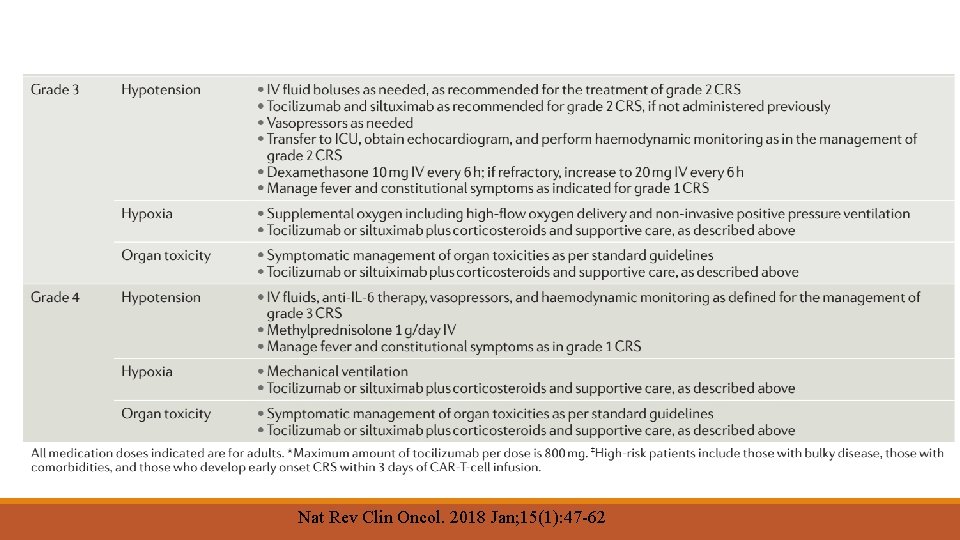
Agents 1. Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel are CD 19 -directed CAR-T immunotherapies that are approved by the US Food and Drug Administration (FDA) for treatment of adults with relapsed or refractory DLBCL after two or more lines of systemic therapy. 2. Both are only available in the United States through a risk evaluation and mitigation strategy (REMS), and the FDA labels carry a boxed warning for CRS and neurologic events. 3. Facilities that dispense these agents require special certification, staff must be trained to recognize and manage adverse events, and tocilizumab (a humanized monoclonal antibody against the interleukin-6 receptor [IL-6 R]) must be available for immediate administration

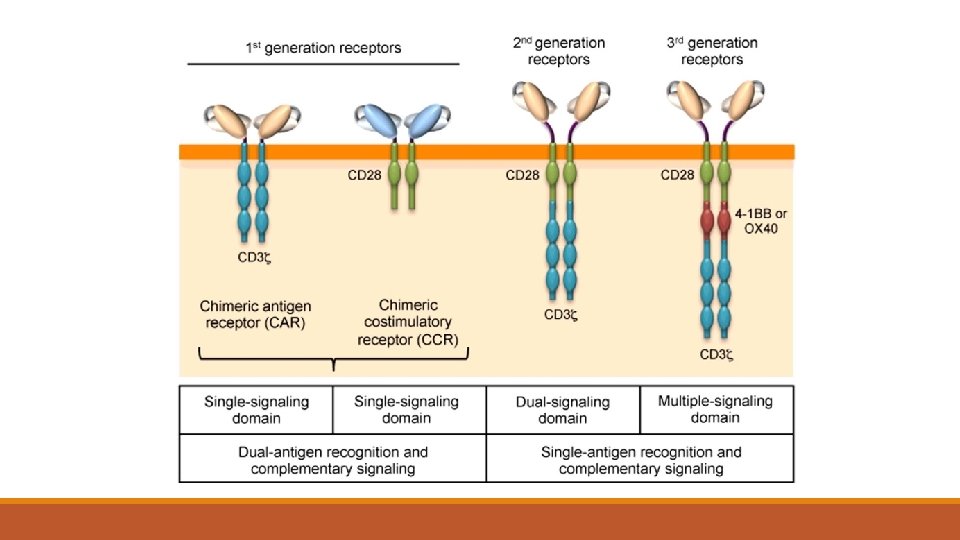
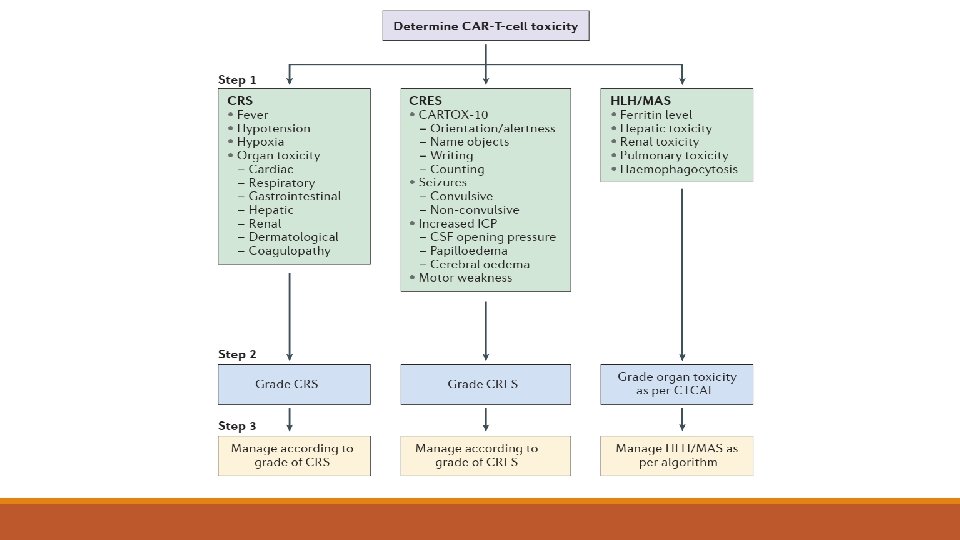
Chimeric antigen receptor T (CAR-T) cells —chimeric antigen receptor T cell therapy (CAR-T), where it is available, based on the balance of clinical benefits, potential long-term disease control, and toxicity




CAR-T cells are a form of genetically modified autologous immunotherapy that have shown activity against DLBCL cells. This customized treatment uses the patient's own T lymphocytes, which are genetically modified (transfected) with a gene that encodes a chimeric antigen receptor to direct the patient's T cells against the lymphoma cells. The T cells are genetically modified ex vivo, expanded in a production facility, and then infused back into the patient as therapy.

Primary study In a single center study, Schuster et al enrolled 28 patients with CD 19+ NHL that had measurable disease after primary and salvage therapies, relapsed disease after autologous stem cell transplantation, or were ineligible for autologous stem cell transplantation Patients received lentiviral-transduced anti-CD 19 CAR-T cells with CD 3ζ and a 4 -1 BB costimulatory domain, termed CTL 019. A variety of lymphodepletion regimens was utilized 14 had DLBCL with an overall response rate (ORR) of 50% at 3 months. At 6 months, 6/14 patients with DLBCL had a CR; however, the median progression-free survival (PFS) for this cohort was only 3. 2 months. CRS occurred in 57% among all treated patients, with 18% grade 3 or higher.

Primary study Neurologic toxicity was observed in 39% of patients, with 11% grade 3 or higher. Despite presence of toxicities, there was minimal utilization of tocilizumab or corticosteroids in this cohort

The JULIET trial A single-arm multicenter, international, pivotal phase 2 clinical trial of tisagenlecleucel (CTL 019) in patients with relapsed/refractory DLBCL. Eligible patients: had received two or more lines of chemotherapy and had either failed autologous stem cell transplant or were transplant ineligible. Bridging chemotherapy was allowed after apheresis as clinically indicated. Lymphodepletion options included bendamustine or a fludarabine/cyclophosphamide combination

Continue From 165 patients enrolled, only 111 (67%) patients received CTL 019 T-cells. ◦ Among the 93 patients evaluable for efficacy, ◦ The ORR was 52% (40% CR and 12% PR). At 3 months, ◦ The complete remission rate was 32% and the PR rate 6%; at 6 months the CR rate was 33%. ◦ The 12 -month probability of being relapse free among responding patients was 65%. ◦ The median OS for patients receiving an infusion was 12 months. CRS occurred in 58% of patients, 22% being grade 3 or higher as assessed by the University of Pennsylvania grading system, and 12% of patients had grade 3 or higher neurologic toxicity

Tocilizumab was utilized in 14% of patients while 10% received both corticosteroids and tocilizumab. These data from JULIET led to the FDA approval of tisagenlecleucel (Kymriah) for adults with relapsed or refractory DLBCL after two or more lines of therapy

ZUMA-1 A phase 2 multicenter clinical trial for axicabtagene ciloleucel (axi-cel) enrolled 111 patients with refractory DLBCL and primary mediastinal B-cell lymphoma (PMBCL) for anti-CD 19 CAR-T cell therapy. Refractory disease was defined as progressive or stable disease to most recent chemotherapy regimen, or disease progression or relapse within 12 months of autologous stem cell transplantation.

ZUMA-1 Eligible patients were treated with axi-cel, a CD 28 CD 3ζ retroviral anti-CD 19 CAR-T cell. Unlike the JULIET trial, patients were not allowed bridging chemotherapy between apheresis and planned CART cell infusion. All patients received fludarabine/cyclophosphamide for lymphodepletion. Of the 111 patients enrolled, 101 received treatment with axi-cel with an objective response rate of 82% and a complete response rate of 54%.

At a median follow-up of 15. 4 months, 40% remain in CR. The most common adverse events were pyrexia, neutropenia, and anemia. CRS occurred in 93% of patients, though most were grade 1 -2 as per the Lee et al CRS grading system (only 13% ≥ grade 3). Neurologic toxicity occurred in 64% of patients, 28% being grade 3 or higher, and resolved in most cases. Tocilizumab was required in 43% of patients and 27% received steroids.

ZUMA-1 led to FDA approval of axicabtagene ciloleucel (Yescarta) for adult patients with relapsed or refractory DLBCL, primary mediastinal B-cell lymphoma, and DLBCL arising from follicular lymphoma (FL) who have failed two or more lines of systemic therapy

TRANSCEND NHL 001, a multicenter phase 1 trial of another candidate anti CD 19 CAR-T cell product, lisocabtagene maraleucel (liso-cel). Lisocel is a CD 19 -directed 4 -1 BB CD 3ζ lentiviral CAR-T cell product. Unlike the other CD 19 CAR-T products, liso-cel is administered with a defined composition of CD 4: CD 8 T cells at a ratio of 1: 1. In this phase 1 study, 69 patients with relapsed/refractory B cell NHL were treated and evaluated for safety, and 68 patients were evaluated for efficacy.

TRANSCEND All patients received a combination of fludarabine/cyclophosphamide for lymphodepletion The ORR at 3 months was 75% and the 6 -month CR rate was 37%. CRS was seen in 30% of patients, and only a single patient (1%) developed severe CRS (grade 4) as per Lee et al CRS scale. Neurotoxicity occurred in 20% of patients, including 14% grade 3 or higher symptoms. For management of these toxicities, 19% received tocilizumab and 9% received dexamethasone




Nat Rev Clin Oncol. 2018 Jan; 15(1): 47 -62

PD-1 inhibition and CAR-T cell therapy Engagement of the PD-1 receptor on T-cells by PD-L 1 leads to inhibition of T-cell activity and is an established mechanism of immune evasion utilized to promote the survival of malignant diseases. Jacobson et al presented results from a phase 1 study of axi-cel in combination with atezolizumab (anti-PD-L 1 antibody) for the treatment of patients with refractory DLBCL.

PD-1 inhibition and CAR-T cell therapy Patients received four doses of atezolizumab starting at varying time points after axi-cel infusion. There was no exacerbation of axi-cel related toxicity seen and among evaluable patients, six were in CR and three were in PR post-infusion. Another approach utilized is to augment CAR-T cell activity with immune checkpoint blockade in patients who relapse after CAR-T cell therapy. In a recently reported clinical trial, patients who failed a 41 BB CD 3ζ anti-CD 19 CAR-T cell product received pembrolizumab (anti-PD 1 antibody) every 3 weeks until progression. Among 11 evaluable patients, only three had a clinical response (one CR and two PR). It remains unclear to date what role immune checkpoint inhibitors will have with CAR-T cell therapy.

396 patients with DLBCL who underwent allogenic HCT following MAC (165 patients), RIC (143 patients), or NMA conditioning (88 patients). When compared with those who underwent RIC and NMA, patients treated with MAC were younger, less likely to have had an autologous HCT, and more likely to have disease that was refractory to treatment and/or of advanced stage. MAC was associated with higher rates of five-year NRM (56 versus 47 and 36 percent, respectively), but lower rates of relapse/progression (26 versus 38 and 40 percent). Estimated five-year rates of PFS (15 to 25 percent) and OS (18 to 26 percent) were similar following the different conditioning regimens

Acute and chronic graft-versus-host disease (GVHD) were seen in 43 and 40 percent, respectively, and were similar following the different conditioning regimens. On multivariate analysis, factors that were associated with worse survival included Karnofsky performance score <90 (HR 1. 48, 95% CI 1. 16 -1. 90) and relapse with chemoresistant disease (HR 1. 70, 95% CI 1. 14 -2. 56)

When car-t therapy is not available, we offer allogenic HCT for patients who are medically eligible.
