NSN 2017 Malmo New expensive treatments Can we









- Slides: 14

NSN 2017, Malmo New expensive treatments: Can we afford them? Chairs: Annette Bruchfeld, Stockholm Runolfur Palsson, Reykjavik NSN 2017, Malmo

Disclosures AB has received consulting fees from Chemo. Centryx and Merck/MSD, and received honoraria for lectures from Abb. Vie, Chemo. Centryx and Merck/MSD RP has nothing to disclose

Rising costs of prescription drugs account for an increasingly large proportion of healthcare expenditures The majority of drugs approved in recent years are very expensive specialty drugs, including drugs for rare diseases (orphan drugs) A marked increase in prices of many older, commonly used medications has also occurred The industry’s primary defense of the rising medicine prices are the high costs associated with drug development


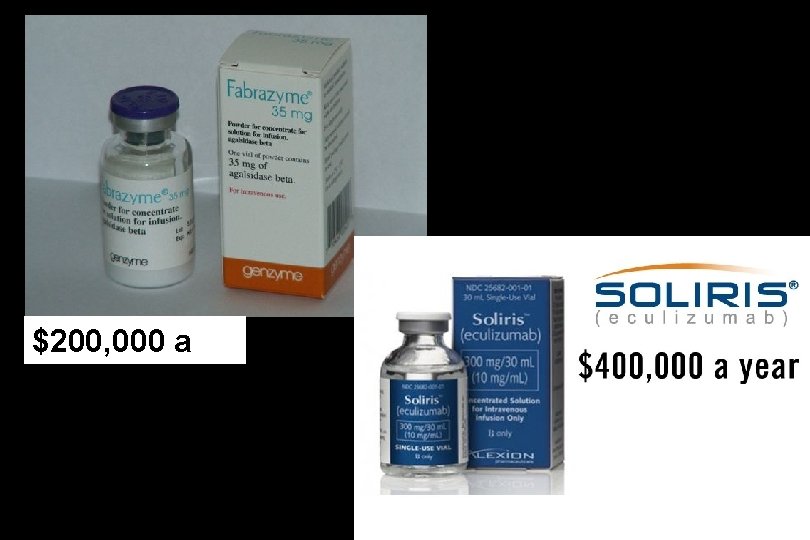
$200, 000 a year

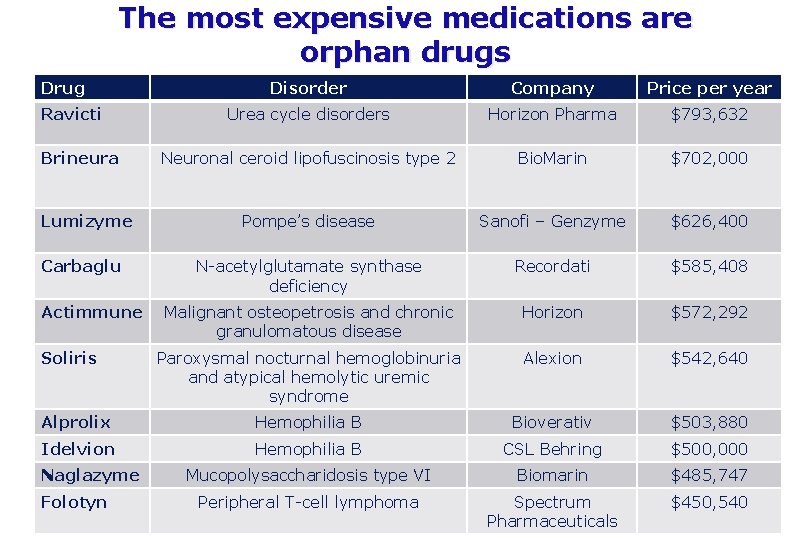
The most expensive medications are orphan drugs Drug Disorder Company Price per year Urea cycle disorders Horizon Pharma $793, 632 Neuronal ceroid lipofuscinosis type 2 Bio. Marin $702, 000 Pompe’s disease Sanofi – Genzyme $626, 400 N-acetylglutamate synthase deficiency Recordati $585, 408 Malignant osteopetrosis and chronic granulomatous disease Horizon $572, 292 Paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome Alexion $542, 640 Alprolix Hemophilia B Bioverativ $503, 880 Idelvion Hemophilia B CSL Behring $500, 000 Mucopolysaccharidosis type VI Biomarin $485, 747 Peripheral T-cell lymphoma Spectrum Pharmaceuticals $450, 540 Ravicti Brineura Lumizyme Carbaglu Actimmune Soliris Naglazyme Folotyn

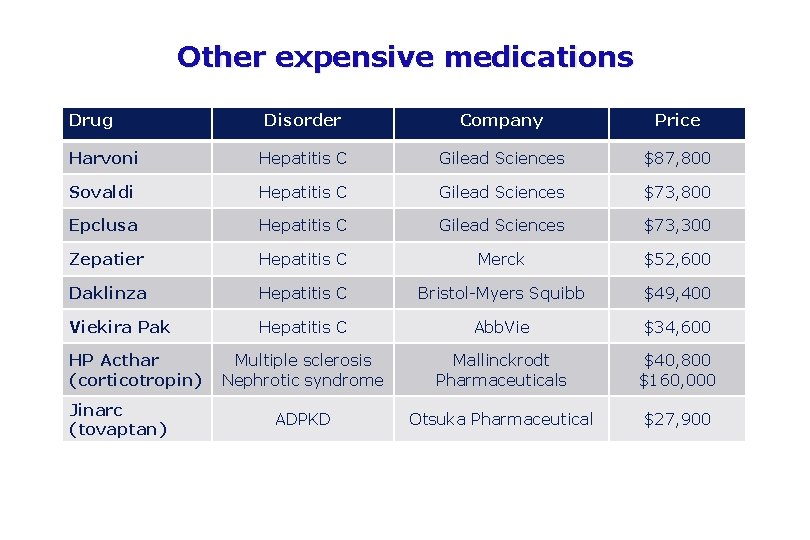
Other expensive medications Drug Disorder Company Price Harvoni Hepatitis C Gilead Sciences $87, 800 Sovaldi Hepatitis C Gilead Sciences $73, 800 Epclusa Hepatitis C Gilead Sciences $73, 300 Zepatier Hepatitis C Merck $52, 600 Daklinza Hepatitis C Bristol-Myers Squibb $49, 400 Viekira Pak Hepatitis C Abb. Vie $34, 600 Multiple sclerosis Nephrotic syndrome Mallinckrodt Pharmaceuticals $40, 800 $160, 000 ADPKD Otsuka Pharmaceutical $27, 900 HP Acthar (corticotropin) Jinarc (tovaptan)

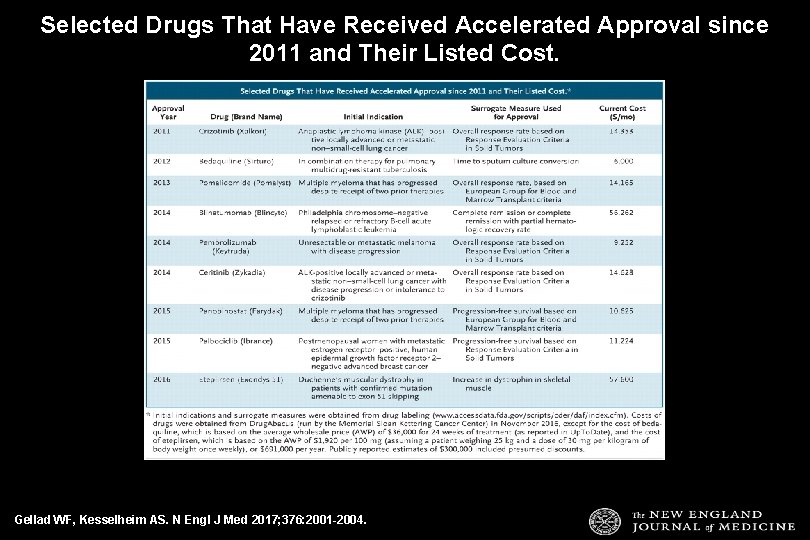
Selected Drugs That Have Received Accelerated Approval since 2011 and Their Listed Cost. Gellad WF, Kesselheim AS. N Engl J Med 2017; 376: 2001 -2004.

Over the period of the study, 76 unique indications were granted without RCT results (44 by the EMA and 60 by the FDA) No systematic monitoring of such treatments to confirm their effectiveness or consistency regarding when this form of evidence is appropriate

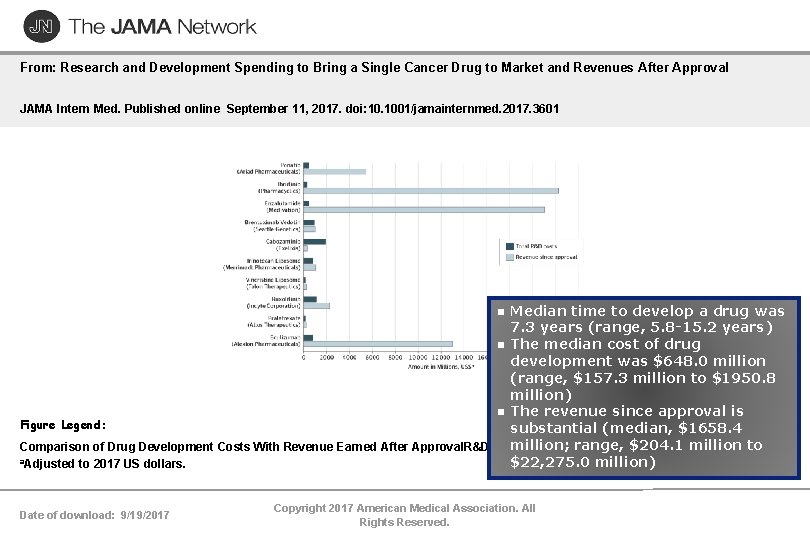
From: Research and Development Spending to Bring a Single Cancer Drug to Market and Revenues After Approval JAMA Intern Med. Published online September 11, 2017. doi: 10. 1001/jamainternmed. 2017. 3601 Median time to develop a drug was 7. 3 years (range, 5. 8 -15. 2 years) The median cost of drug development was $648. 0 million (range, $157. 3 million to $1950. 8 million) The revenue since approval is Figure Legend: substantial (median, $1658. 4 million; range, $204. 1 million to Comparison of Drug Development Costs With Revenue Earned After Approval. R&D indicates research and development. a. Adjusted to 2017 US dollars. $22, 275. 0 million) Date of download: 9/19/2017 Copyright 2017 American Medical Association. All Rights Reserved.

Action is needed If authorities of Western countries do not take action, the cost of pharmaceutical agents would be predicted to rise by at least 10% every year, eventually leading to unsustainable expenditures for the healthcare system Clearly, a solution of this challenging problem is needed


Panelists 1. Lars Sandman, Council for New Therapies, Sweden 2. Steinar Madsen, Medical Director, Norwegian Medicines Agency 3. Douglas Lundin, Dental and Pharmaceutical Benefits Agency, Sweden 4. Søren Rittig, Pediatric Nephrologist, Professor of Pediatrics, Aarhus University, Denmark 5. Åsa Magnusson, Commission for Innovative and Rare Pharmaceuticals, Sweden 6. Taina Mäntyranta, General Secretary, Ministry of Social Affairs and Health and Council for Choices in Healthcare, Finland

Questions for the panelists 1. What are the main drivers of increasing pharmaceutical expenditures? 2. How is this problem being addressed in your country? 3. Is access to expensive orphan drugs limited in your country? Should the access be unlimited irrespective of price or should there be a cost-effectiveness threshold? 4. What is the responsibility of pharmaceutical companies regarding prescription drug prices? 5. Should physicians' prescriptions of expensive medications be controlled? 6. What strategies do you propose to control the rising pharmaceutical costs?