VARIOUS WATER TREATMENTS VARIOUS WATER TREATMENTS Primary Water












- Slides: 17

VARIOUS WATER TREATMENTS

VARIOUS WATER TREATMENTS Primary Water Treatment Technologies 1. Screening, Filtration and Centrifugal Separation 2. Sedimentation and Gravity Separation 3. Coagulation 4. Floatation SECONDARY WATER TREATMENT 1. Aerobic Process 2. Anaerobic Process

Tertiary Water Treatment 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Distillation Evaporation Oxidation Ion exchange Reverse Osmosis Crystallization Solvent Extraction Precipitation Micro – and Ultra Filtration Adsorption Electrodialysis

ION EXCHANGE RESINS CATION EXCHANGE RESINS ANION EXCHANGE RESINS PROCESS REGENERATION

ION EXCHANGE ION-EXCHANGE RESINS : Insoluble Cross-linked, long chain organic polymers Micro porous structure Functional groups attached to the chains are responsible for the ion-exchanging properties

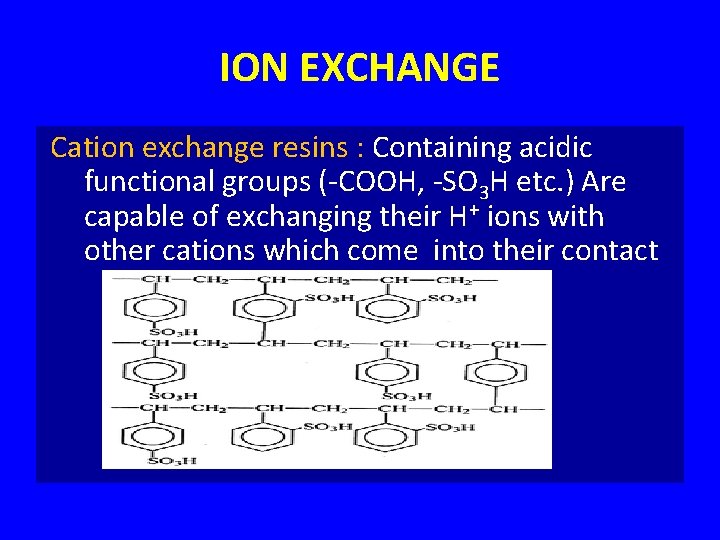
ION EXCHANGE Cation exchange resins : Containing acidic functional groups (-COOH, -SO 3 H etc. ) Are capable of exchanging their H+ ions with other cations which come into their contact

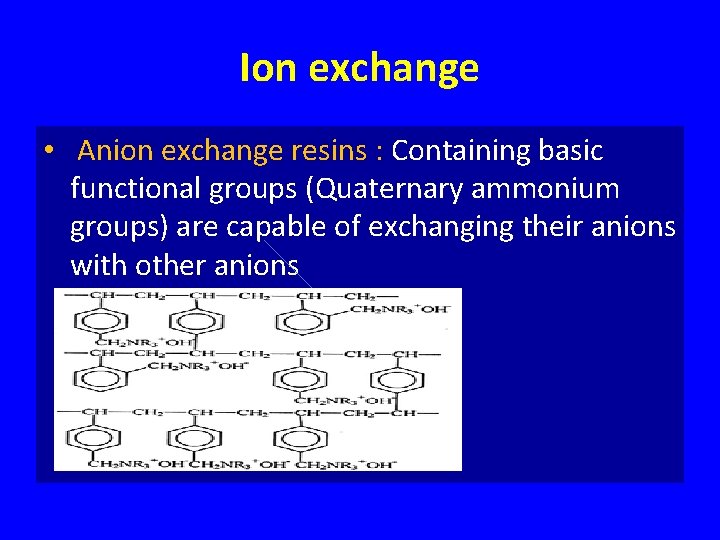
Ion exchange • Anion exchange resins : Containing basic functional groups (Quaternary ammonium groups) are capable of exchanging their anions with other anions

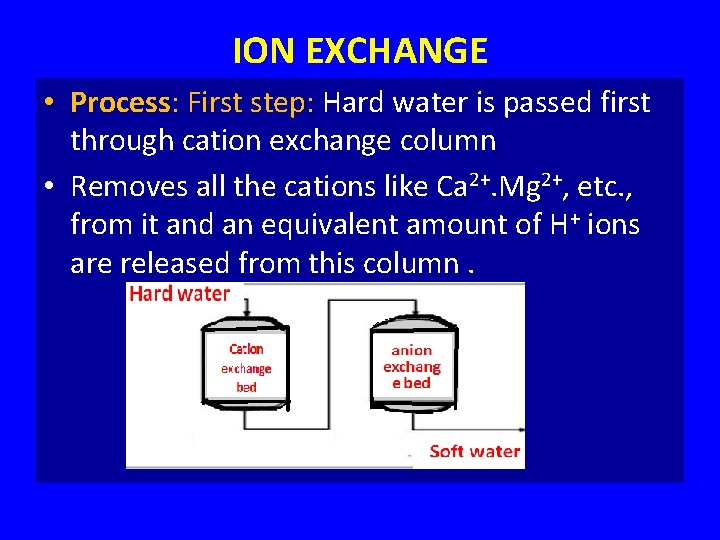
ION EXCHANGE • Process: First step: Hard water is passed first through cation exchange column • Removes all the cations like Ca 2+. Mg 2+, etc. , from it and an equivalent amount of H+ ions are released from this column.

ION EXCHANGE Process: Second step • Water which is now free from cations, is passed through anion exchange column • Removes all the anions like SO 42 -, cl- etc. , Present in the water and equivalent amount OH- ions are released from this column to water


Ion exchange • H+ and OH- ions (released from cation exchange and anion exchange columns respectively) combine to produce water. • Thus the water coming out from the exchanger is free from all cations as well as anions. Ion-free water is known as de ionised or de mineralised water.

ION EXCHANGE • Regeneration: • When capacities of cation and anion exchangers to exchange H+ and OH- ions respectively are lost, they are then said to be exhausted. • The exhausted cation exchange column is regenerated by passing a solution of dil. HCl or H 2 SO 4.

Reverse osmosis • SEMI PERMEABLE MEMBRANE • OSMOSIS • REVERSE OSMOSIS

• Semi permeable membrane : It is biological or synthetic, polymeric membrane that will allow certain molecules or ions. • E. g Lipid layer • Thin film membrane that lines the inside of an egg • Polyamide

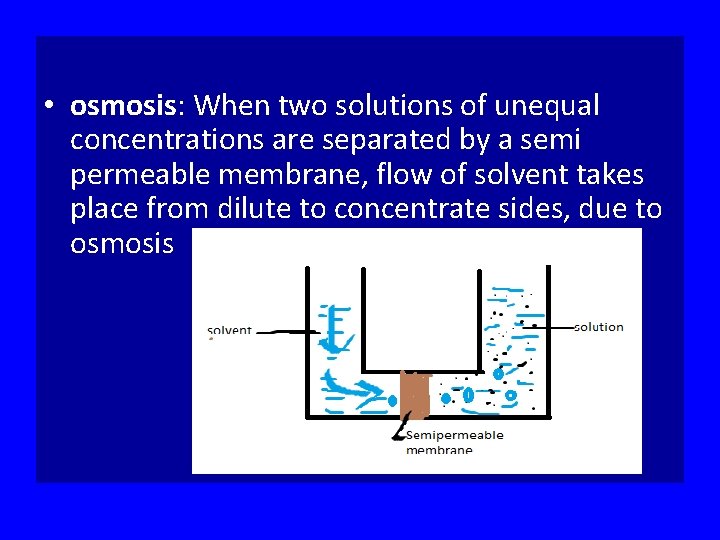
• osmosis: When two solutions of unequal concentrations are separated by a semi permeable membrane, flow of solvent takes place from dilute to concentrate sides, due to osmosis

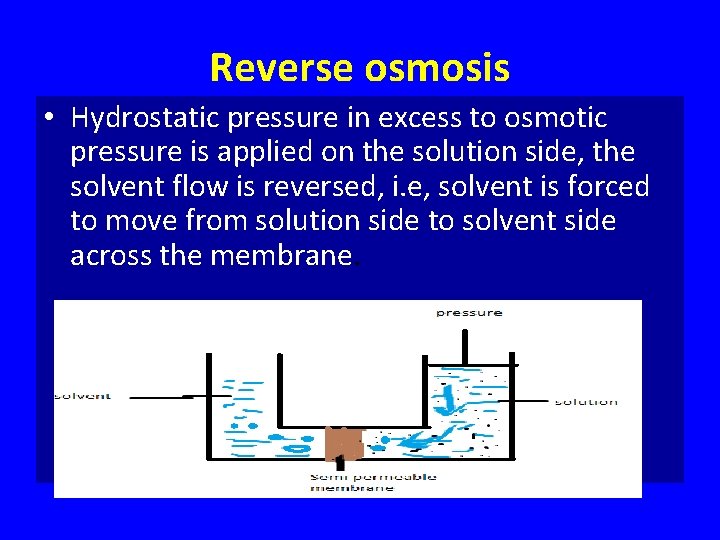
Reverse osmosis • Hydrostatic pressure in excess to osmotic pressure is applied on the solution side, the solvent flow is reversed, i. e, solvent is forced to move from solution side to solvent side across the membrane.

Reverse osmosis principle • Thus in reverse osmosis method, pure solvent is separated from its contaminants, rather than removing contaminants from the water. super-filtration or hyper-filtration.
 Water and water and water water
Water and water and water water Treatments for acute renal failure
Treatments for acute renal failure Level 3 electrical facial
Level 3 electrical facial Alternating treatment
Alternating treatment Hiv treatments
Hiv treatments Diabetes treatments
Diabetes treatments Advanced surface treatments
Advanced surface treatments Insomnia treatment
Insomnia treatment Dementia treatments and interventions near patterson
Dementia treatments and interventions near patterson Magenta cyan and yellow are the ____ color. *
Magenta cyan and yellow are the ____ color. * A website is a collection of web pages
A website is a collection of web pages Alteration in various aspect of society over time
Alteration in various aspect of society over time Explain various boundary descriptors.
Explain various boundary descriptors. Body positions nursing
Body positions nursing Example of concupiscence in ethics
Example of concupiscence in ethics 6 literary lenses
6 literary lenses Digital mechanical calculator
Digital mechanical calculator Various stages of growth are
Various stages of growth are