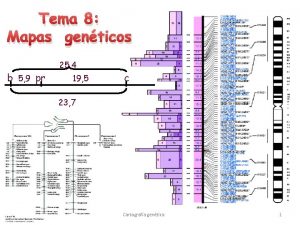
Transcriptional Regulation of Eukaryotic Genes 1 Transcriptional initiation
















- Slides: 52

Transcriptional Regulation of Eukaryotic Genes (真核基因的��� 控) 1. Transcriptional initiation 2. Histone modification 3. DNA methylation Post-Transcriptional Regulation of Eukaryotic Genes (真核基因的�� 后� 控) • RNA silencing (si. RNA and mi. RNA)

Gene Silencing Heterochromatin DNA methylation and histone modifications RNA interference (si. RNA and mi. RNA)

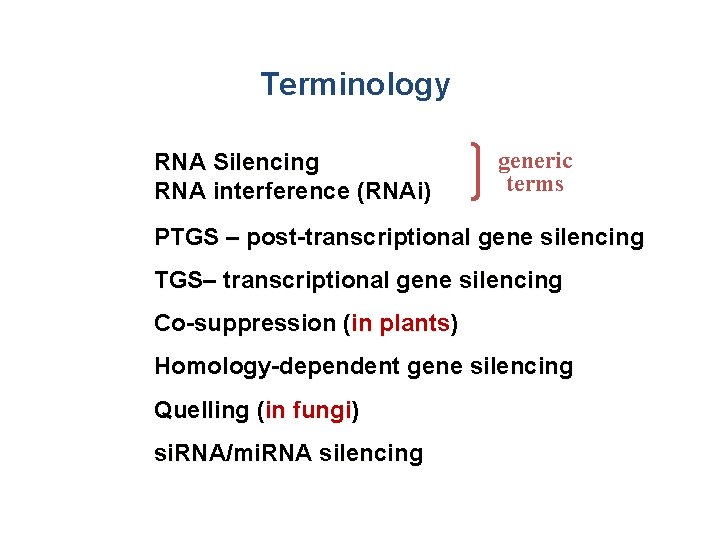
Terminology RNA Silencing RNA interference (RNAi) generic terms PTGS – post-transcriptional gene silencing TGS– transcriptional gene silencing Co-suppression (in plants) Homology-dependent gene silencing Quelling (in fungi) si. RNA/mi. RNA silencing

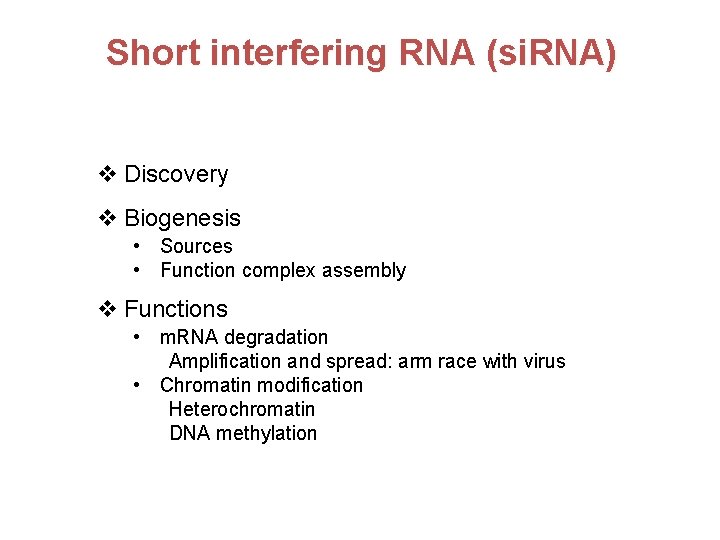
Short interfering RNA (si. RNA) v Discovery v Biogenesis • Sources • Function complex assembly v Functions • m. RNA degradation Amplification and spread: arm race with virus • Chromatin modification Heterochromatin DNA methylation

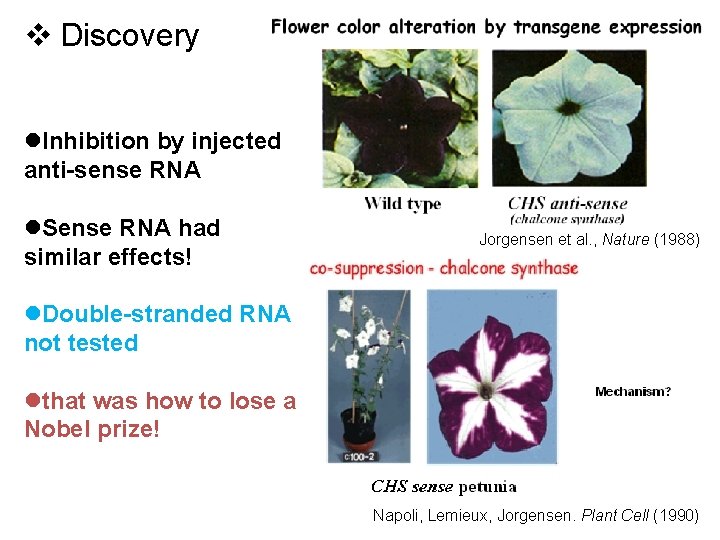
v Discovery l. Inhibition by injected anti-sense RNA l. Sense RNA had similar effects! Jorgensen et al. , Nature (1988) l. Double-stranded RNA not tested lthat was how to lose a Nobel prize! Napoli, Lemieux, Jorgensen. Plant Cell (1990)

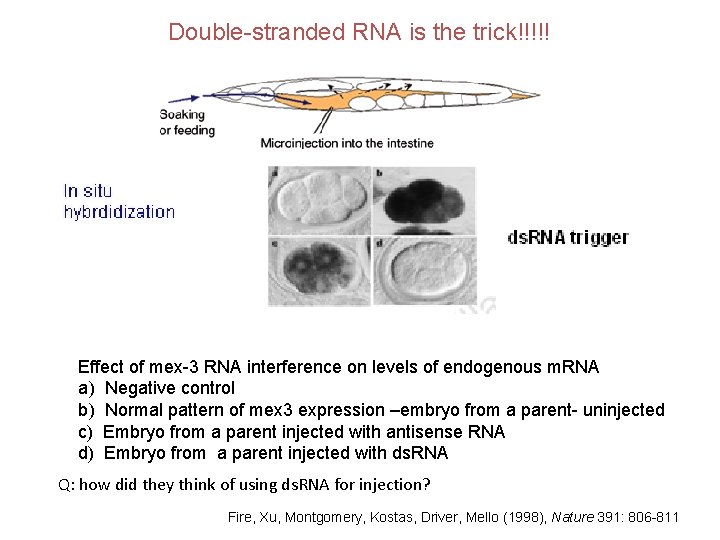
Double-stranded RNA is the trick!!!!! Effect of mex-3 RNA interference on levels of endogenous m. RNA a) Negative control b) Normal pattern of mex 3 expression –embryo from a parent- uninjected c) Embryo from a parent injected with antisense RNA d) Embryo from a parent injected with ds. RNA Q: how did they think of using ds. RNA for injection? Fire, Xu, Montgomery, Kostas, Driver, Mello (1998), Nature 391: 806 -811


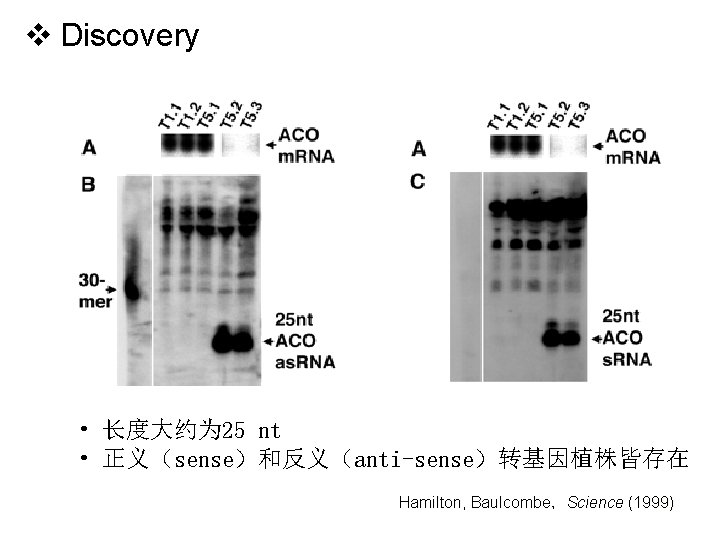
v Discovery • 长度大约为 25 nt • 正义(sense)和反义(anti-sense)转基因植株皆存在 Hamilton, Baulcombe,Science (1999)

For the discovery of RNA interference – gene silencing by ds. RNA and small RNA David Baulcombe

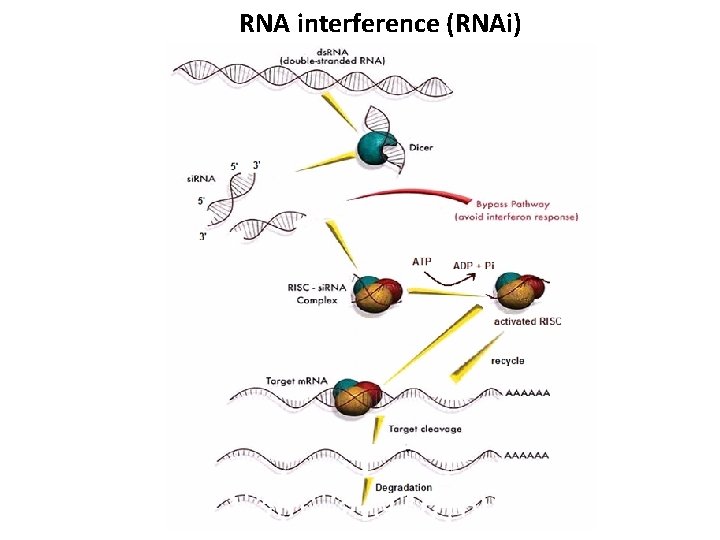
RNA interference (RNAi)

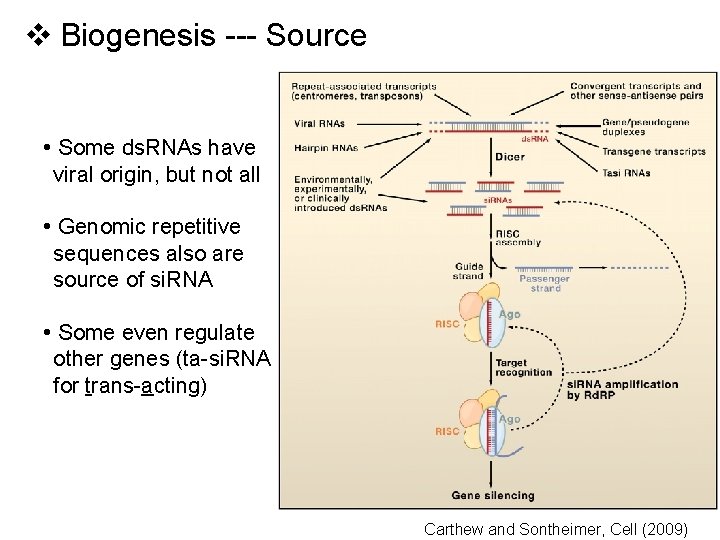
v Biogenesis --- Source • Some ds. RNAs have viral origin, but not all • Genomic repetitive sequences also are source of si. RNA • Some even regulate other genes (ta-si. RNA for trans-acting) Carthew and Sontheimer, Cell (2009)


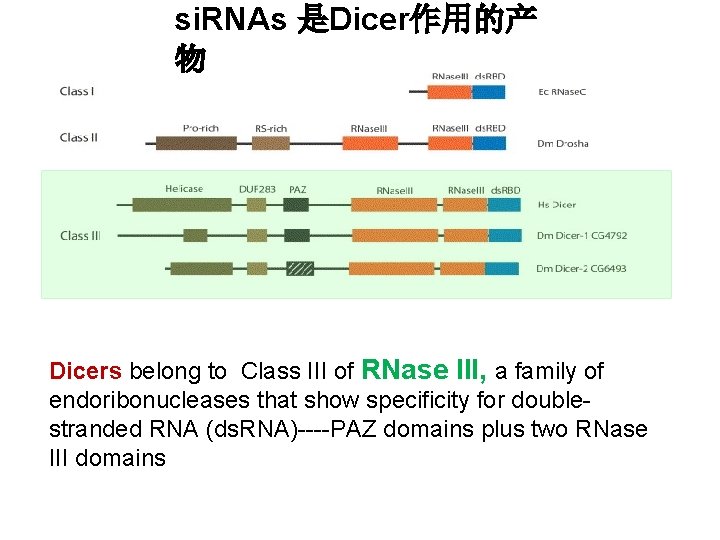
si. RNAs 是Dicer作用的产 物 Dicers belong to Class III of RNase III, a family of endoribonucleases that show specificity for doublestranded RNA (ds. RNA)----PAZ domains plus two RNase III domains

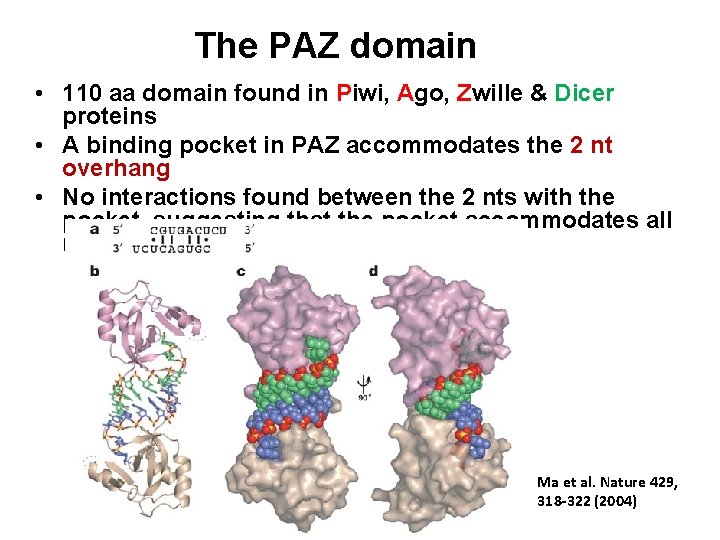
The PAZ domain • 110 aa domain found in Piwi, Ago, Zwille & Dicer proteins • A binding pocket in PAZ accommodates the 2 nt overhang • No interactions found between the 2 nts with the pocket, suggesting that the pocket accommodates all nucleotide combinations Ma et al. Nature 429, 318 -322 (2004)

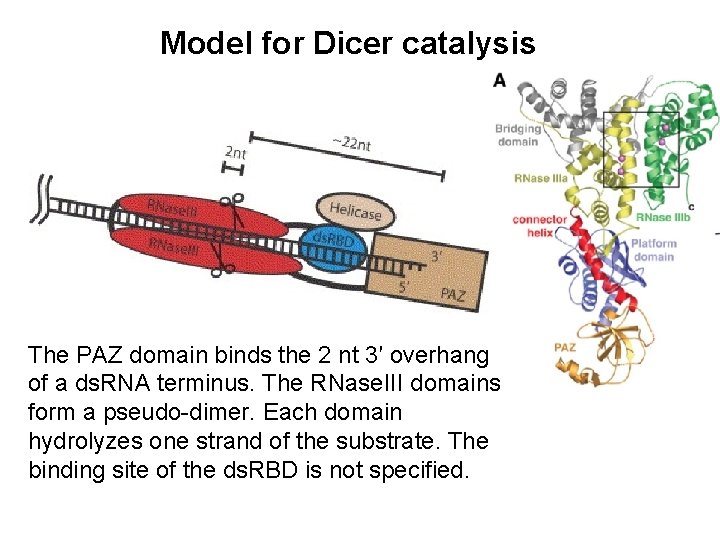
Model for Dicer catalysis The PAZ domain binds the 2 nt 3′ overhang of a ds. RNA terminus. The RNase. III domains form a pseudo-dimer. Each domain hydrolyzes one strand of the substrate. The binding site of the ds. RBD is not specified.

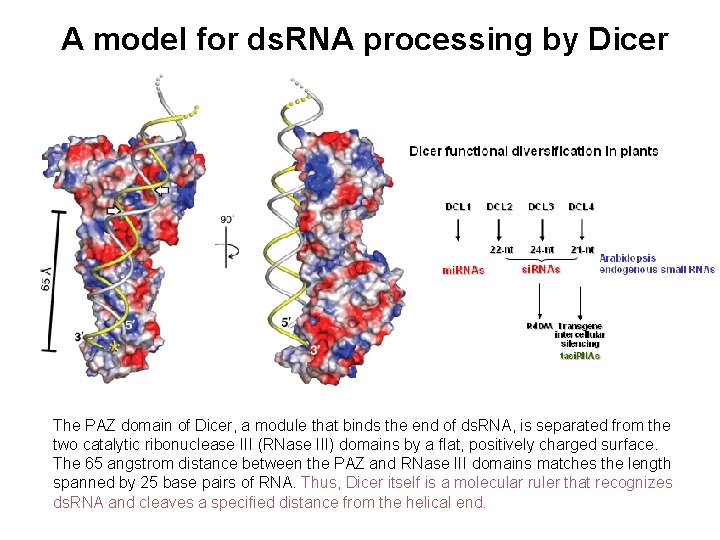
A model for ds. RNA processing by Dicer The PAZ domain of Dicer, a module that binds the end of ds. RNA, is separated from the two catalytic ribonuclease III (RNase III) domains by a flat, positively charged surface. The 65 angstrom distance between the PAZ and RNase III domains matches the length spanned by 25 base pairs of RNA. Thus, Dicer itself is a molecular ruler that recognizes ds. RNA and cleaves a specified distance from the helical end.

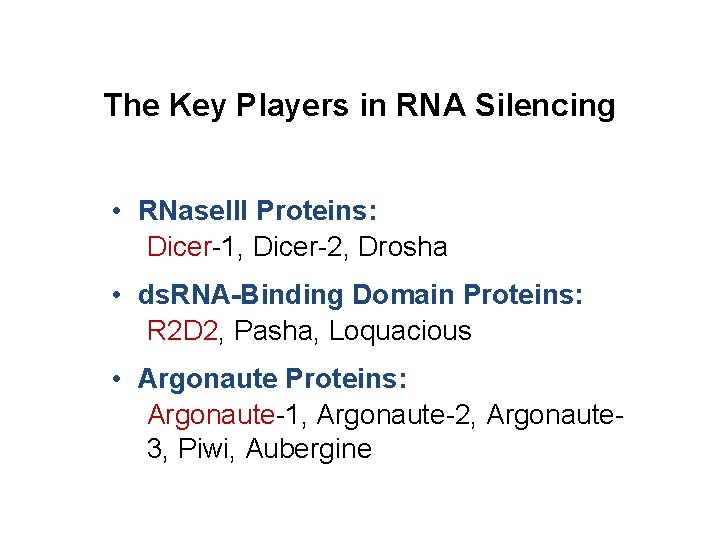
The Key Players in RNA Silencing • RNase. III Proteins: Dicer-1, Dicer-2, Drosha • ds. RNA-Binding Domain Proteins: R 2 D 2, Pasha, Loquacious • Argonaute Proteins: Argonaute-1, Argonaute-2, Argonaute 3, Piwi, Aubergine


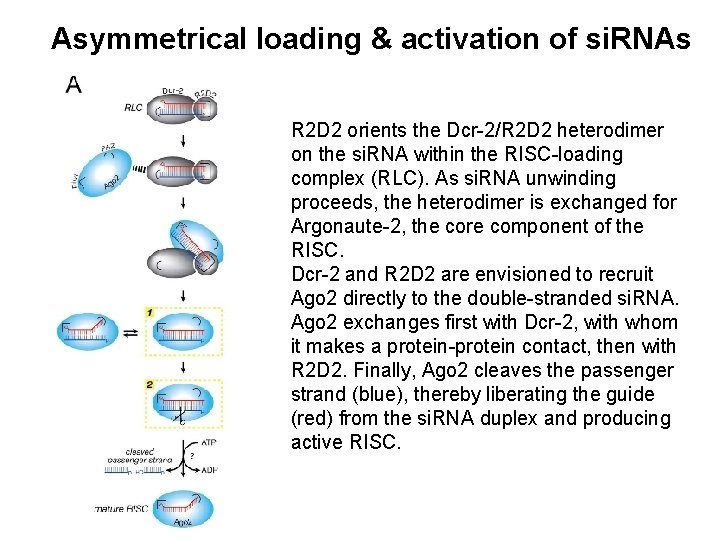
Asymmetrical loading & activation of si. RNAs R 2 D 2 orients the Dcr-2/R 2 D 2 heterodimer on the si. RNA within the RISC-loading complex (RLC). As si. RNA unwinding proceeds, the heterodimer is exchanged for Argonaute-2, the core component of the RISC. Dcr-2 and R 2 D 2 are envisioned to recruit Ago 2 directly to the double-stranded si. RNA. Ago 2 exchanges first with Dcr-2, with whom it makes a protein-protein contact, then with R 2 D 2. Finally, Ago 2 cleaves the passenger strand (blue), thereby liberating the guide (red) from the si. RNA duplex and producing active RISC.

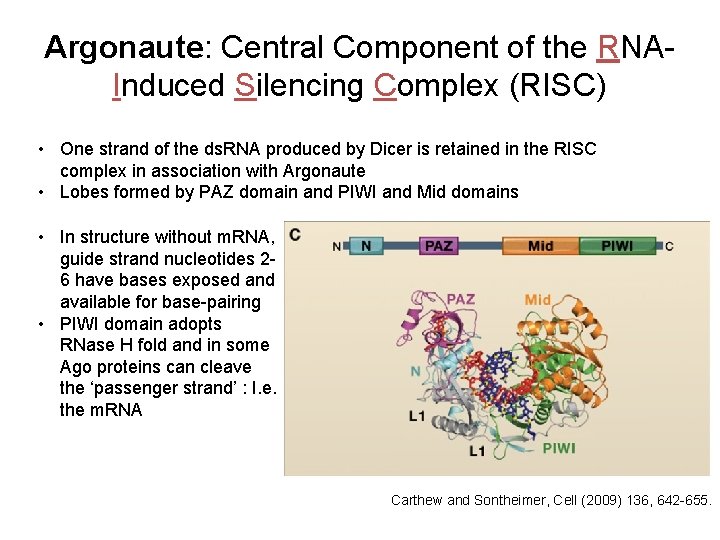
Argonaute: Central Component of the RNAInduced Silencing Complex (RISC) • One strand of the ds. RNA produced by Dicer is retained in the RISC complex in association with Argonaute • Lobes formed by PAZ domain and PIWI and Mid domains • In structure without m. RNA, guide strand nucleotides 26 have bases exposed and available for base-pairing • PIWI domain adopts RNase H fold and in some Ago proteins can cleave the ‘passenger strand’ : I. e. the m. RNA Carthew and Sontheimer, Cell (2009) 136, 642 -655.

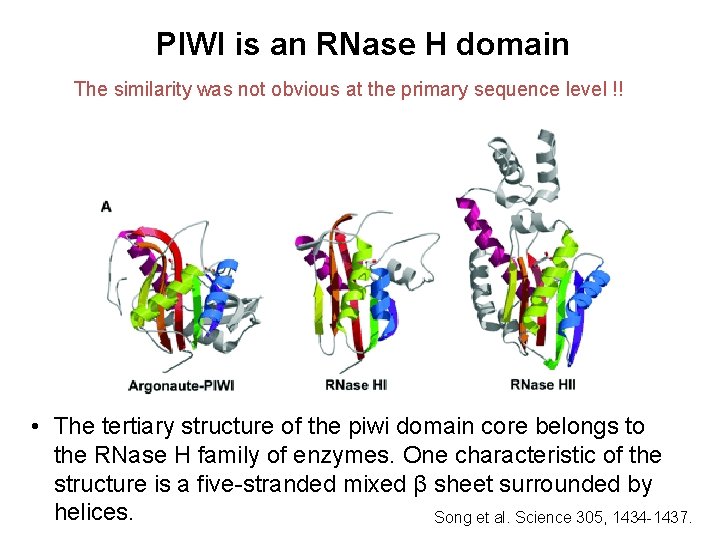
PIWI is an RNase H domain The similarity was not obvious at the primary sequence level !! • The tertiary structure of the piwi domain core belongs to the RNase H family of enzymes. One characteristic of the structure is a five-stranded mixed β sheet surrounded by helices. Song et al. Science 305, 1434 -1437.

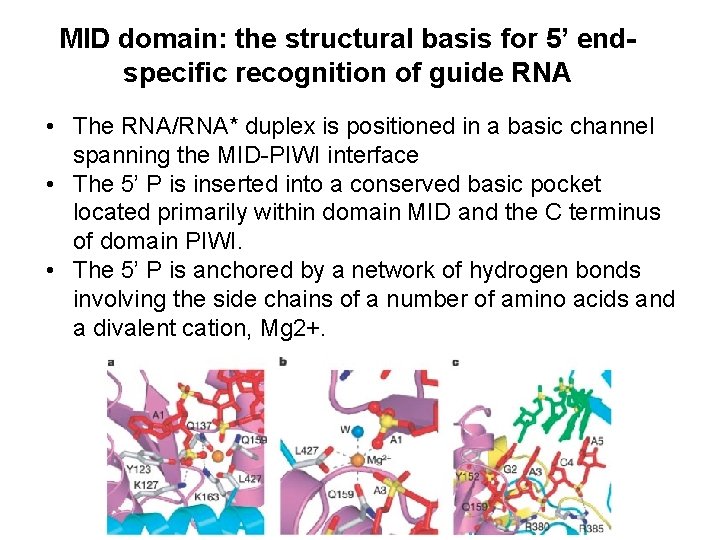
MID domain: the structural basis for 5’ endspecific recognition of guide RNA • The RNA/RNA* duplex is positioned in a basic channel spanning the MID-PIWI interface • The 5’ P is inserted into a conserved basic pocket located primarily within domain MID and the C terminus of domain PIWI. • The 5’ P is anchored by a network of hydrogen bonds involving the side chains of a number of amino acids and a divalent cation, Mg 2+.

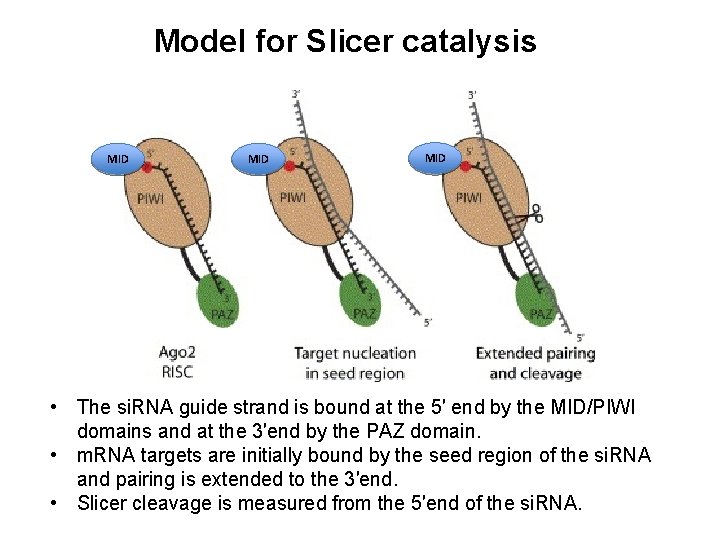
Model for Slicer catalysis MID MID • The si. RNA guide strand is bound at the 5′ end by the MID/PIWI domains and at the 3′end by the PAZ domain. • m. RNA targets are initially bound by the seed region of the si. RNA and pairing is extended to the 3′end. • Slicer cleavage is measured from the 5′end of the si. RNA.

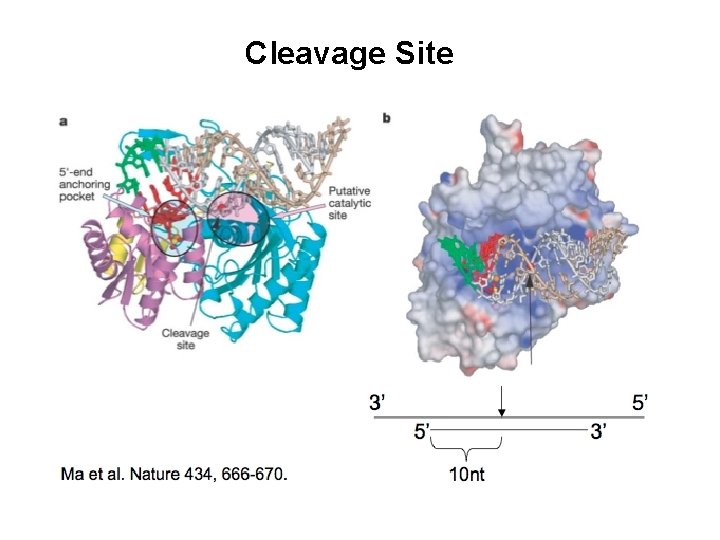
Cleavage Site

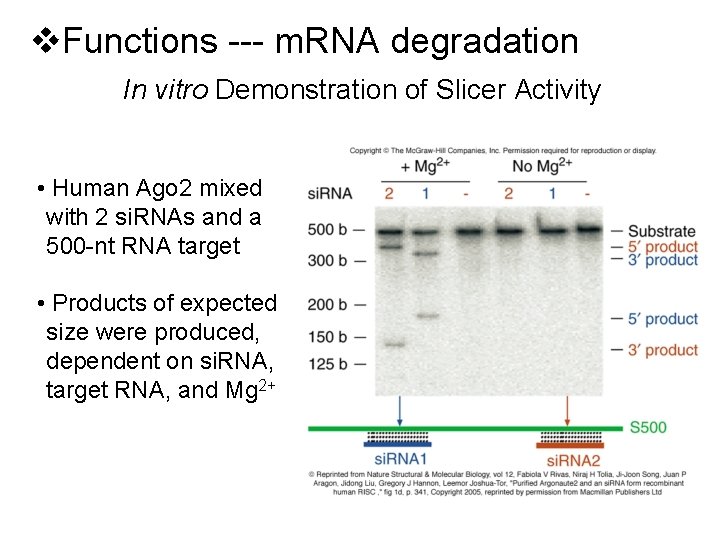
v. Functions --- m. RNA degradation In vitro Demonstration of Slicer Activity • Human Ago 2 mixed with 2 si. RNAs and a 500 -nt RNA target • Products of expected size were produced, dependent on si. RNA, target RNA, and Mg 2+

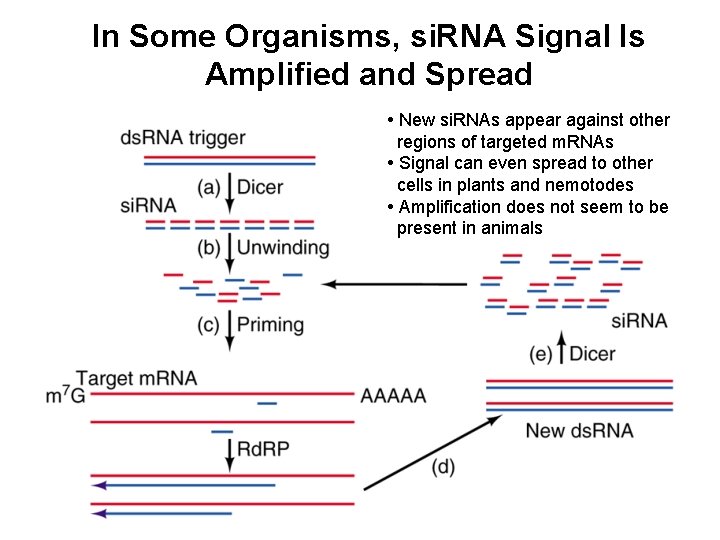
In Some Organisms, si. RNA Signal Is Amplified and Spread • New si. RNAs appear against other regions of targeted m. RNAs • Signal can even spread to other cells in plants and nemotodes • Amplification does not seem to be present in animals


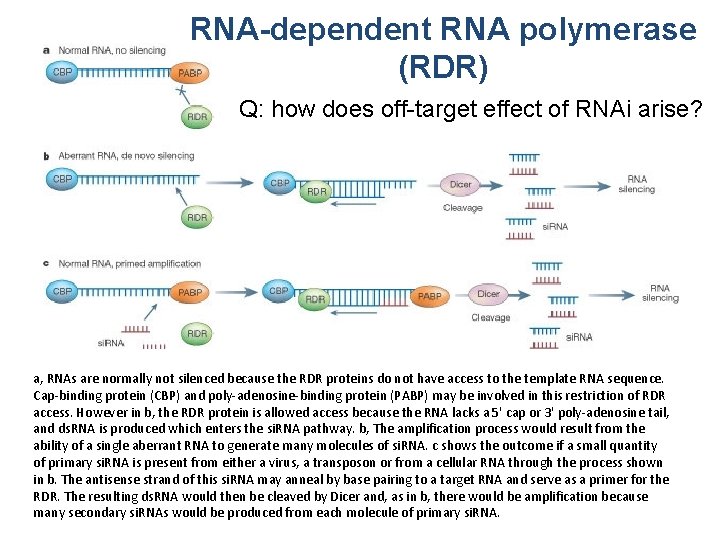
RNA-dependent RNA polymerase (RDR) Q: how does off-target effect of RNAi arise? a, RNAs are normally not silenced because the RDR proteins do not have access to the template RNA sequence. Cap-binding protein (CBP) and poly-adenosine-binding protein (PABP) may be involved in this restriction of RDR access. However in b, the RDR protein is allowed access because the RNA lacks a 5' cap or 3' poly-adenosine tail, and ds. RNA is produced which enters the si. RNA pathway. b, The amplification process would result from the ability of a single aberrant RNA to generate many molecules of si. RNA. c shows the outcome if a small quantity of primary si. RNA is present from either a virus, a transposon or from a cellular RNA through the process shown in b. The antisense strand of this si. RNA may anneal by base pairing to a target RNA and serve as a primer for the RDR. The resulting ds. RNA would then be cleaved by Dicer and, as in b, there would be amplification because many secondary si. RNAs would be produced from each molecule of primary si. RNA.


Short and long range cell to cell movement of RNAi


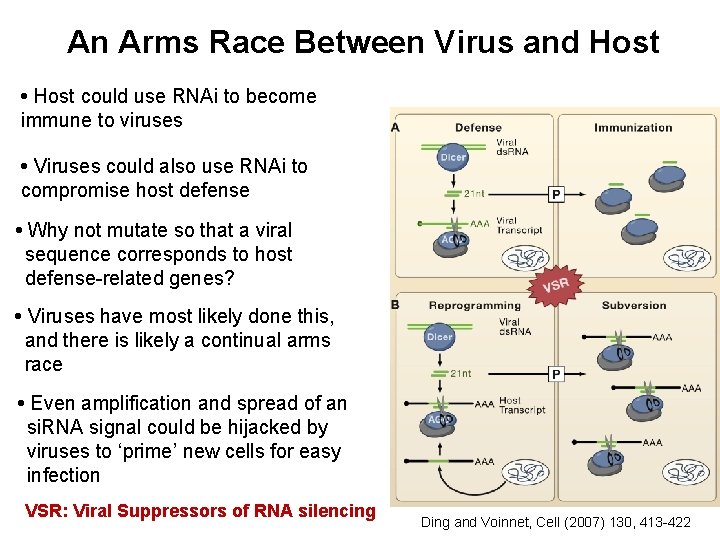
An Arms Race Between Virus and Host • Host could use RNAi to become immune to viruses • Viruses could also use RNAi to compromise host defense • Why not mutate so that a viral sequence corresponds to host defense-related genes? • Viruses have most likely done this, and there is likely a continual arms race • Even amplification and spread of an si. RNA signal could be hijacked by viruses to ‘prime’ new cells for easy infection VSR: Viral Suppressors of RNA silencing Ding and Voinnet, Cell (2007) 130, 413 -422

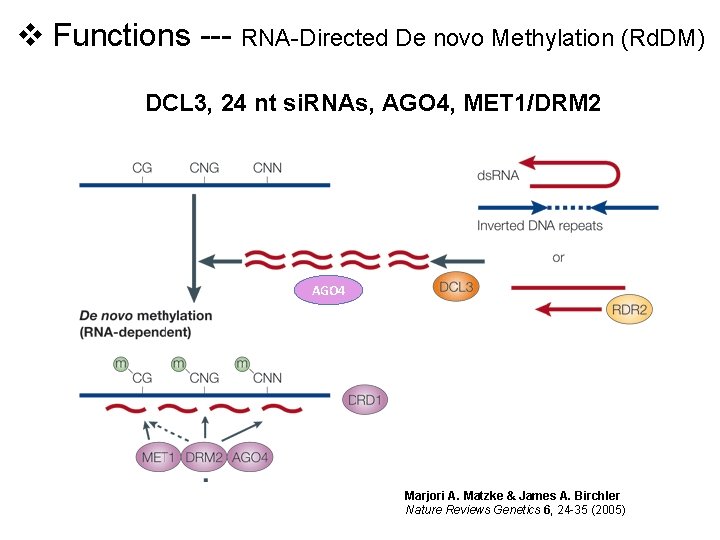
v Functions --- RNA-Directed De novo Methylation (Rd. DM) DCL 3, 24 nt si. RNAs, AGO 4, MET 1/DRM 2 AGO 4 Marjori A. Matzke & James A. Birchler Nature Reviews Genetics 6, 24 -35 (2005)


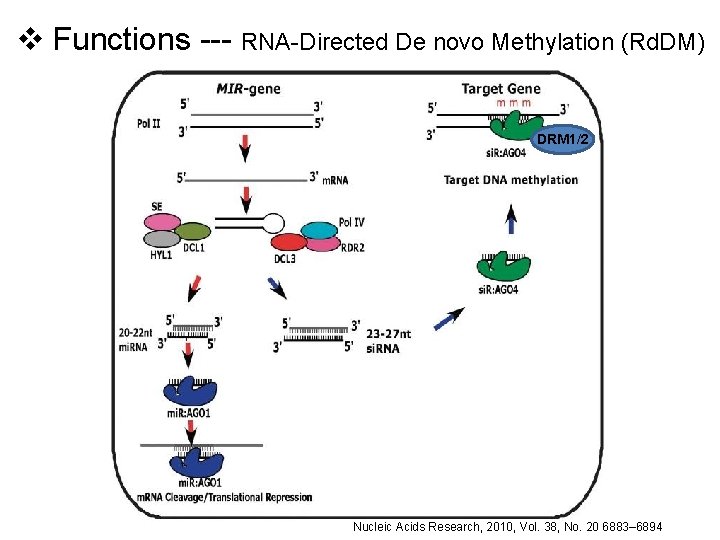
v Functions --- RNA-Directed De novo Methylation (Rd. DM) DRM 1/2 Nucleic Acids Research, 2010, Vol. 38, No. 20 6883– 6894

Micro RNA (mi. RNA) v Discovery v Biogenesis • Complex loading selection v Compare with si. RNA v Functions • m. RNA degradation • Ribosome drop-off • Initiation block v Technical application • Artificial mi. RNA



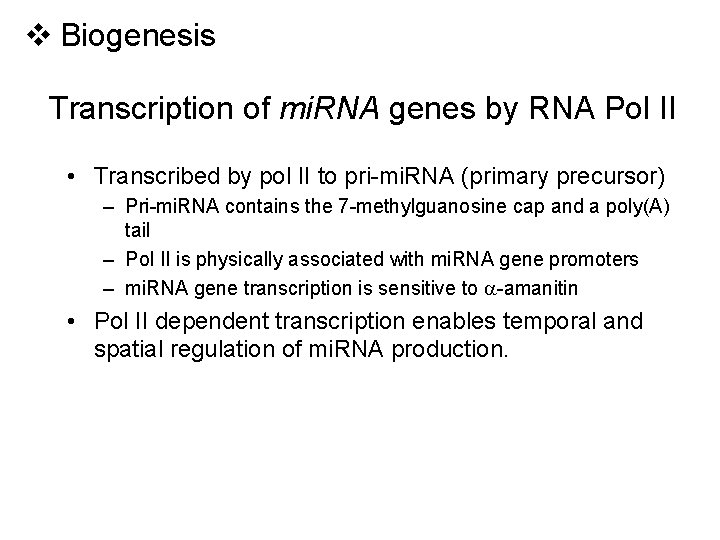
v Biogenesis Transcription of mi. RNA genes by RNA Pol II • Transcribed by pol II to pri-mi. RNA (primary precursor) – Pri-mi. RNA contains the 7 -methylguanosine cap and a poly(A) tail – Pol II is physically associated with mi. RNA gene promoters – mi. RNA gene transcription is sensitive to -amanitin • Pol II dependent transcription enables temporal and spatial regulation of mi. RNA production.

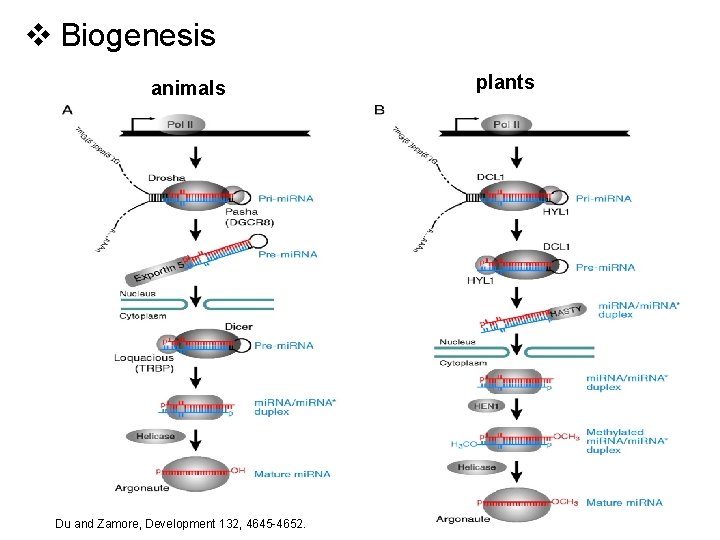
v Biogenesis animals Du and Zamore, Development 132, 4645 -4652. plants

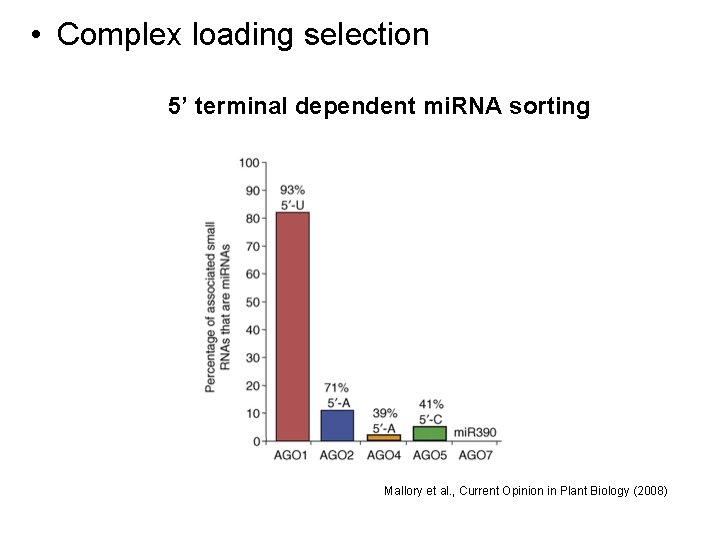
• Complex loading selection 5’ terminal dependent mi. RNA sorting Mallory et al. , Current Opinion in Plant Biology (2008)

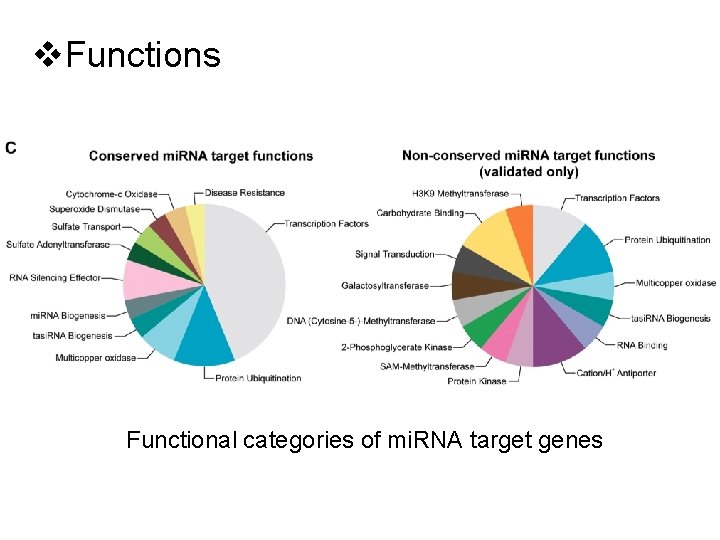
v. Functions Functional categories of mi. RNA target genes

micro. RNA的作用机理 • Reduction of m. RNA stability – Plant mi. RNAs guide cleavage of target m. RNAs (however only a few mi. RNA targets have been examined at the protein level) – Animal mi. RNAs also reduce stability of target m. RNAs • Inhibition of m. RNA translation – lin-4 mediated regulation of lin-14; let-7 mediated regulation of lin-41 – Other animal mi. RNAs cause reduced target protein levels without affecting target m. RNA levels in cell culture – At least three examples of plant mi. RNAs affecting target protein but not m. RNA levels

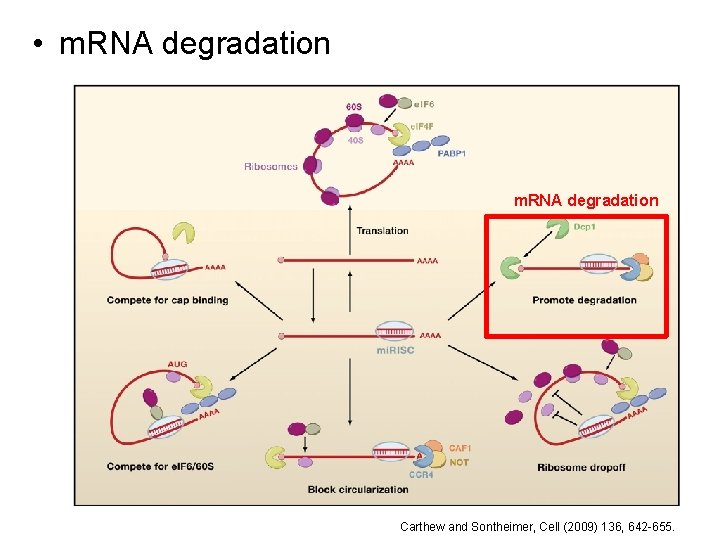
• m. RNA degradation Carthew and Sontheimer, Cell (2009) 136, 642 -655.

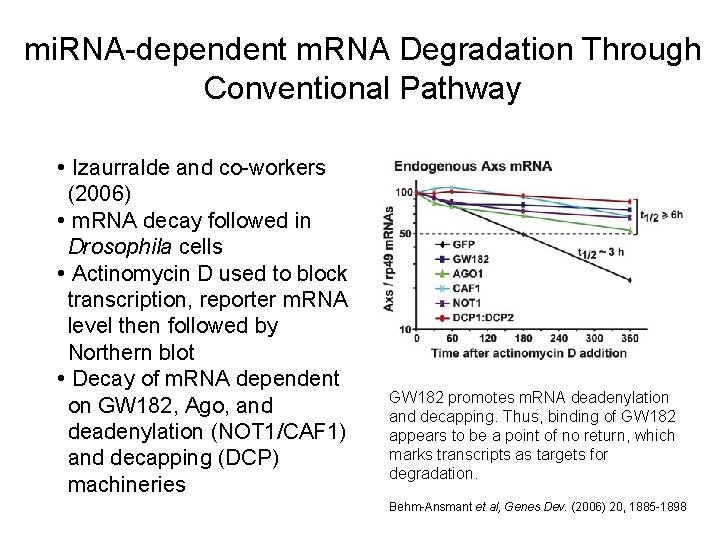
mi. RNA-dependent m. RNA Degradation Through Conventional Pathway • Izaurralde and co-workers (2006) • m. RNA decay followed in Drosophila cells • Actinomycin D used to block transcription, reporter m. RNA level then followed by Northern blot • Decay of m. RNA dependent on GW 182, Ago, and deadenylation (NOT 1/CAF 1) and decapping (DCP) machineries GW 182 promotes m. RNA deadenylation and decapping. Thus, binding of GW 182 appears to be a point of no return, which marks transcripts as targets for degradation. Behm-Ansmant et al, Genes Dev. (2006) 20, 1885 -1898

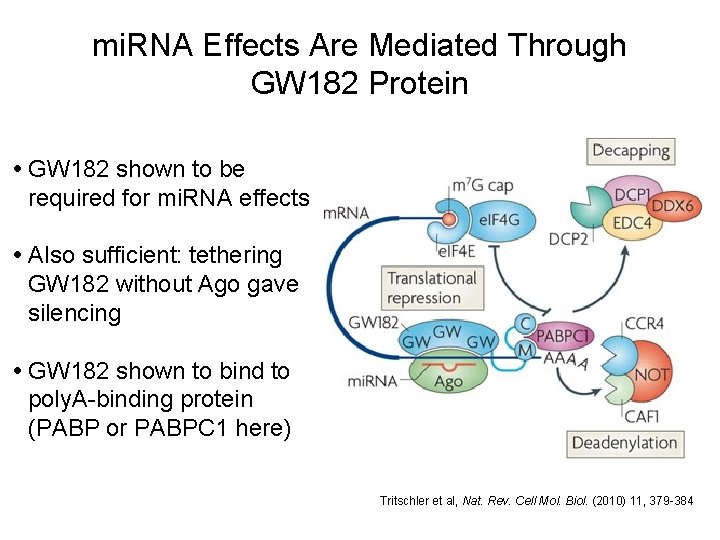
mi. RNA Effects Are Mediated Through GW 182 Protein • GW 182 shown to be required for mi. RNA effects • Also sufficient: tethering GW 182 without Ago gave silencing • GW 182 shown to bind to poly. A-binding protein (PABP or PABPC 1 here) Tritschler et al, Nat. Rev. Cell Mol. Biol. (2010) 11, 379 -384

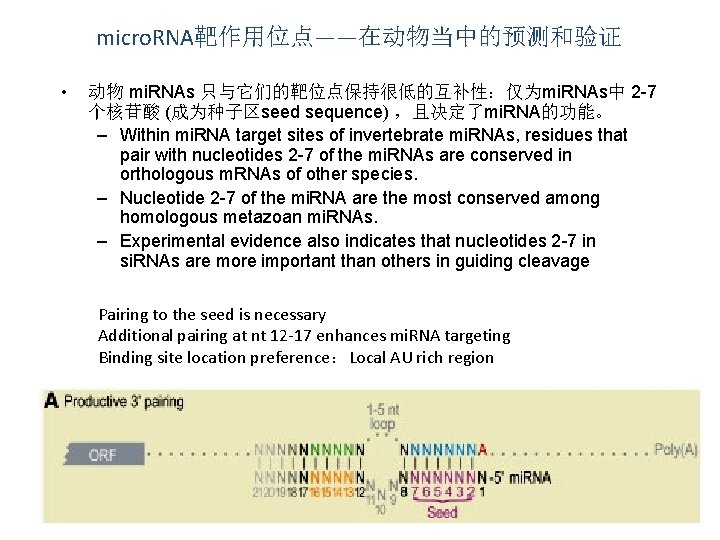
micro. RNA靶作用位点——在动物当中的预测和验证 • 动物 mi. RNAs 只与它们的靶位点保持很低的互补性:仅为mi. RNAs中 2 -7 个核苷酸 (成为种子区seed sequence) ,且决定了mi. RNA的功能。 – Within mi. RNA target sites of invertebrate mi. RNAs, residues that pair with nucleotides 2 -7 of the mi. RNAs are conserved in orthologous m. RNAs of other species. – Nucleotide 2 -7 of the mi. RNA are the most conserved among homologous metazoan mi. RNAs. – Experimental evidence also indicates that nucleotides 2 -7 in si. RNAs are more important than others in guiding cleavage Pairing to the seed is necessary Additional pairing at nt 12 -17 enhances mi. RNA targeting Binding site location preference:Local AU rich region


mi. RNA的生物学功能 • • Plant mi. RNAs – Many act in cell differentiation and developmental patterning by targeting transcription factor m. RNAs – Other mi. RNAs target non-transcription factor m. RNAs and may play a role in physiological processes or stress responses – Essential functions of mi. RNAs illustrated by the embryo lethal phenotype of dcl 1 null mutants Animal mi. RNAs – Developmental patterning • ES cells lacking Dicer are viable but cannot differentiate in vitro and in vivo. • Dicer knockout zebrafish lacking both maternal and zygotic Dicer have intact patterning in the first 24 h but fail to continue with morphogenesis – Physiological functions – Cancer

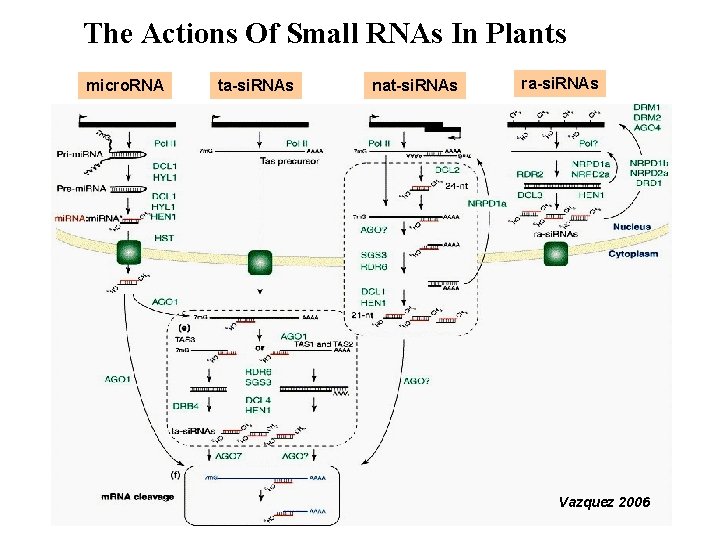
The Actions Of Small RNAs In Plants micro. RNA ta-si. RNAs nat-si. RNAs ra-si. RNAs Vazquez 2006

Key Points • Small RNAs, in the forms of si. RNAs and mi. RNAs, play large roles in the regulation of gene expression in eukaryotes. They are important in normal cell metabolism, development, and defense against invaders. • si. RNAs are produced from longer segments of ds. RNA by Dicer, assembed into RISC, and targeted to m. RNAs with perfect complementarity, giving silencing by cleavage and degradation of the RNA or by formation of heterochromatin. • The pathway for mi. RNA has many steps in common with that for si. RNA. However, mi. RNAs are processed from hairpin structures by Drosha and then by Dicer, and they most often have imperfect complementarity with their targets, giving effects on translation rather than Ago-mediated cleavage of the m. RNA.

 Linked genes and unlinked genes
Linked genes and unlinked genes Homeotic genes
Homeotic genes Polygenic inheritance
Polygenic inheritance Ccc 1359
Ccc 1359 Sop sign off sheet
Sop sign off sheet In file organization a fixed format is used for records
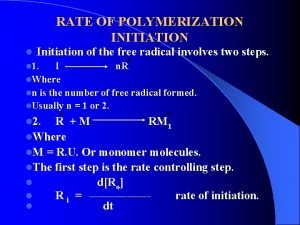
In file organization a fixed format is used for records Initiation radical reaction
Initiation radical reaction Pre initiation phase project management
Pre initiation phase project management Simultaneous transcription
Simultaneous transcription A quoi sert la qualité
A quoi sert la qualité Site initiation visit in clinical trials ppt
Site initiation visit in clinical trials ppt Clozapine titration chart
Clozapine titration chart Hero's journey revelation
Hero's journey revelation Sensibilisation et initiation à la cybersécurité
Sensibilisation et initiation à la cybersécurité Divergent diagram
Divergent diagram Raci 차트
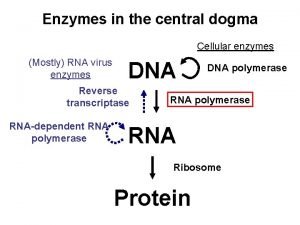
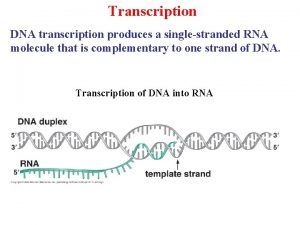
Raci 차트 Dna central dogma
Dna central dogma Topic initiation
Topic initiation L'initiation
L'initiation Contoh project initiation
Contoh project initiation Transcription initiation in eukaryotes
Transcription initiation in eukaryotes What is the revelation in the hero journey
What is the revelation in the hero journey Knapp's stages of coming together
Knapp's stages of coming together Importance of project initiation
Importance of project initiation Project identification and initiation
Project identification and initiation Initiation promotion progression
Initiation promotion progression Initiation à la démonstration 5ème
Initiation à la démonstration 5ème Phases of home visit in community health nursing
Phases of home visit in community health nursing The task archetype examples
The task archetype examples What is the ordeal in the odyssey
What is the ordeal in the odyssey Energy amplifier initiation
Energy amplifier initiation Initiation and maintenance of callus culture
Initiation and maintenance of callus culture Initiation à la recherche en soins infirmiers
Initiation à la recherche en soins infirmiers Formulacion de la teoria cromosomica de la herencia
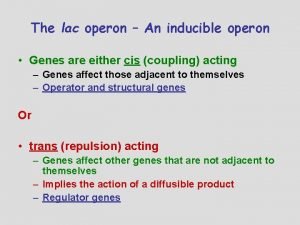
Formulacion de la teoria cromosomica de la herencia Lac operon
Lac operon Evolution of populations section 16-1 genes and variation
Evolution of populations section 16-1 genes and variation Genes ligados
Genes ligados Heterozygous recessive
Heterozygous recessive This section describes
This section describes Punnett square example
Punnett square example How genes work
How genes work Complementary genes in sweet pea
Complementary genes in sweet pea Genes located on the sex chromosomes
Genes located on the sex chromosomes Dna and genes chapter 11
Dna and genes chapter 11 Linked genes
Linked genes Jumping genes
Jumping genes Non protein coding genes
Non protein coding genes Punnet square blood type
Punnet square blood type Bill nye genes youtube
Bill nye genes youtube Cancer de colon
Cancer de colon A lac repressor turns off the lac genes by
A lac repressor turns off the lac genes by Oncogenes and tumor suppressor genes
Oncogenes and tumor suppressor genes Dna chromosomes genes diagram
Dna chromosomes genes diagram