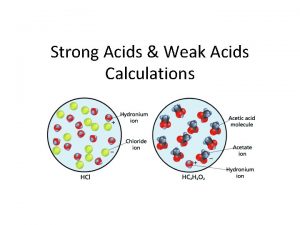
Theory of Indicators Indicators are mainly weak acids



![Indicator Colour • • • [In-] / [HIn] = 0. 1 or less HIn Indicator Colour • • • [In-] / [HIn] = 0. 1 or less HIn](https://slidetodoc.com/presentation_image_h/86b78ec8f1a8b1df84ec2b8c08cf6679/image-4.jpg)


- Slides: 8

Theory of Indicators • Indicators are mainly weak acids or bases • When dissolved in water an equilibrium is reached • • HIn + H 2 O = H 3 O+ + In. HIn is one colour and In- is another In litmus HIn is red and In- is blue When both present it is a mixture of red and blue i. e. purple

Addition of Acid • • H 3 O+ goes up System tries to get it down - Le Chatelier By using up H 3 O+ I. e going left - forming HIn conc goes up. Red colour gets stronger. In- goes down so blue gets weaker Eventually only HIn colour can be seen Litmus is now red

Addition of Base • • Adding OHOH- reacts with H 3 O+ forming 2 H 2 O H 3 O+ goes down System tries to get it back up - Le Chatelier By forming H 3 O+ and In- [going to right] In- conc goes up so blue colour gets stronger HIn goes down so red gets weaker Eventually only In- can be seen - so litmus blue
![Indicator Colour In HIn 0 1 or less HIn Indicator Colour • • • [In-] / [HIn] = 0. 1 or less HIn](https://slidetodoc.com/presentation_image_h/86b78ec8f1a8b1df84ec2b8c08cf6679/image-4.jpg)
Indicator Colour • • • [In-] / [HIn] = 0. 1 or less HIn colour shows [In-] / [HIn] = 10 or more In- colour shows Whichever concentration is 10 times greater - its colour will show • Otherwise intermediate colour

Which Indicator? • • SAWBMO Strong Acid Weak Base Methyl Orange WASBPH Weak Acid Strong Base Phenolphthalein SASBANY Strong Acid Strong Base ANY WAWBNONE Weak Acid Weak Base NONE

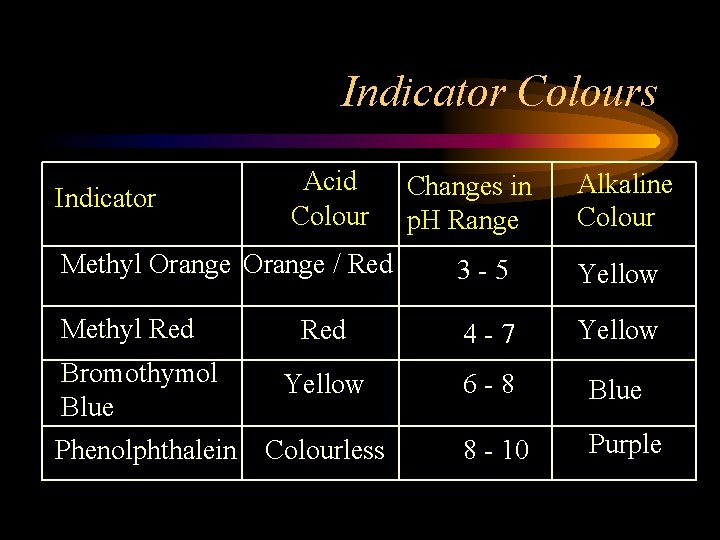
Indicator Colours Indicator Acid Colour Changes in p. H Range Alkaline Colour Methyl Orange / Red 3 -5 Yellow Methyl Red 4 -7 Yellow Bromothymol Blue Yellow 6 -8 Blue Phenolphthalein Colourless 8 - 10 Purple

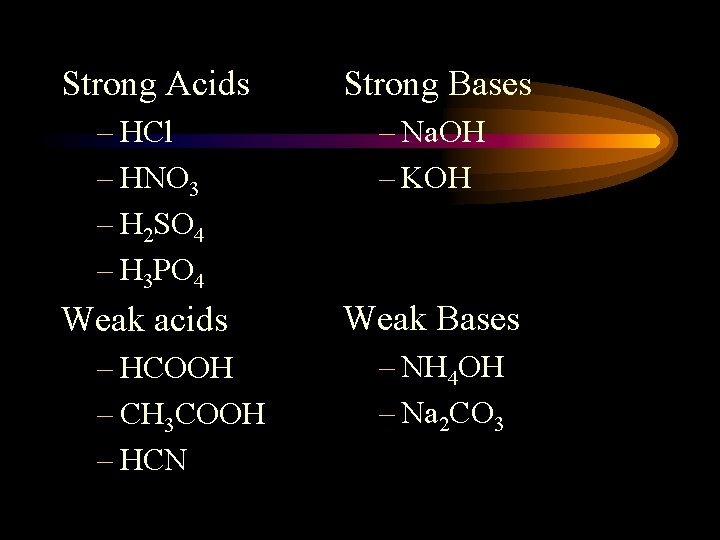
Strong Acids Strong Bases – HCl – HNO 3 – H 2 SO 4 – H 3 PO 4 – Na. OH – KOH Weak acids Weak Bases – HCOOH – CH 3 COOH – HCN – NH 4 OH – Na 2 CO 3

THE END