The 3 rd adverse drug reaction ADR awareness













- Slides: 17

The 3 rd adverse drug reaction (ADR) awareness week campaign: 19 -23 November 2018 Reporting side effects helps the safe use of medicines for babies, children and pregnant women #medsafetyweek Campaign guide for stakeholders and partners

Introduction • Medicines regulators across Europe are running a third social media campaign to increase ADR (adverse drug reaction reports) by patients, public and healthcare professionals between 19 -23 November 2018. • 27 regulators participated in last year’s campaign and evaluation showed ADR reporting increased by 11% across Europe and 16% in the UK. The campaign animations were viewed by 295, 000 people on Twitter, Facebook, Linked. In and You. Tube social media channels. • This year we are running a generic reporting campaign but the emphasis is on encouraging parents/carers and healthcare professionals to report adverse drug reactions in babies and children and expectant mothers to get advice and understand safer medicines during pregnancy. • We have developed three new, humorous animations which focus on these key messages. • We are working with the UN and UNICEF to link the campaign to Universal Children’s Day on 20/11/18 • This guide has been designed to assist stakeholders and partners who wish to support this campaign and its important public health messages. • It explains the objectives, key messages and tactics that regulators across Europe will be supporting during the week including a day-by-day running order of tweets and animations • We have developed a template news release that regulators will issue on 16 November under embargo to 19 November. We will also share a teaser tweet on 16 November about the campaign.

About the campaign • Objective: Raise awareness of how to report ADRs and increase the quality and quantity of reports to provide improved data and a larger data pool for signal detection • Key message: Reporting suspected side-effects makes medicines safer and helps save lives. You can report on www. mhra. gov. uk/yellowcard • Audiences: Parents, carers, patients, public, healthcare professionals • Channels: Social media – Linked. In, Twitter, Facebook, You. Tube, websites, email newsletters

Delivering the campaign • The campaign will run across Europe between 19 -23 November. • 25 member states and three non-European countries are actively supporting the campaign • We are also working closely with the Upsala Monitoring Centre (part of the World Health Organization) who have developed this year’s new animations for us and who will be widening the use of them in the future in African and Latin American countries. • We will also use some of the material we developed last year to support our messages. • All the social media material has been translated in to member state languages, branded with their logos, and has links to their online reporting systems.

How you can support the campaign • If you don’t already, please follow the MHRA’s twitter feeds (MHRA Meds Safety, MHRA News Centre, MHRA Gov, and our other social media channels, Facebook, Instagram and Linked. In • Over the course of the week, retweets and links to the animation and infographics from us or send out your own tweets using the animations we have sent • Provide links or embed our animation in your website. Please let us know if you want to do this and we’ll send them to you. • Send your own messages out on your social media channels, bringing your followers’ attention to the campaign and animation (hosted on You. Tube) • Consider contributing your own perspective / thoughts to the discussion using #medsafetyweek • Tell your members / supporters about the campaign and ask them to support it by sharing/retweeting the links to the animation and infographics via their personal Linked. In, Twitter, email newsletter and other professional networks etc. • Provide us with feedback on the engagement you have generated via your social media channels so we can feed into our overall evaluation report

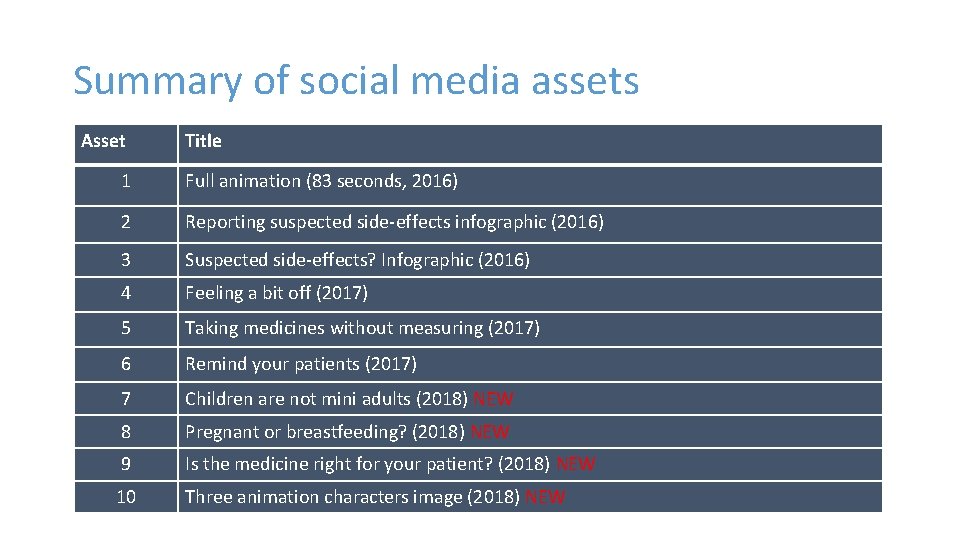
Summary of social media assets Asset Title 1 Full animation (83 seconds, 2016) 2 Reporting suspected side-effects infographic (2016) 3 Suspected side-effects? Infographic (2016) 4 Feeling a bit off (2017) 5 Taking medicines without measuring (2017) 6 Remind your patients (2017) 7 Children are not mini adults (2018) NEW 8 Pregnant or breastfeeding? (2018) NEW 9 Is the medicine right for your patient? (2018) NEW 10 Three animation characters image (2018) NEW

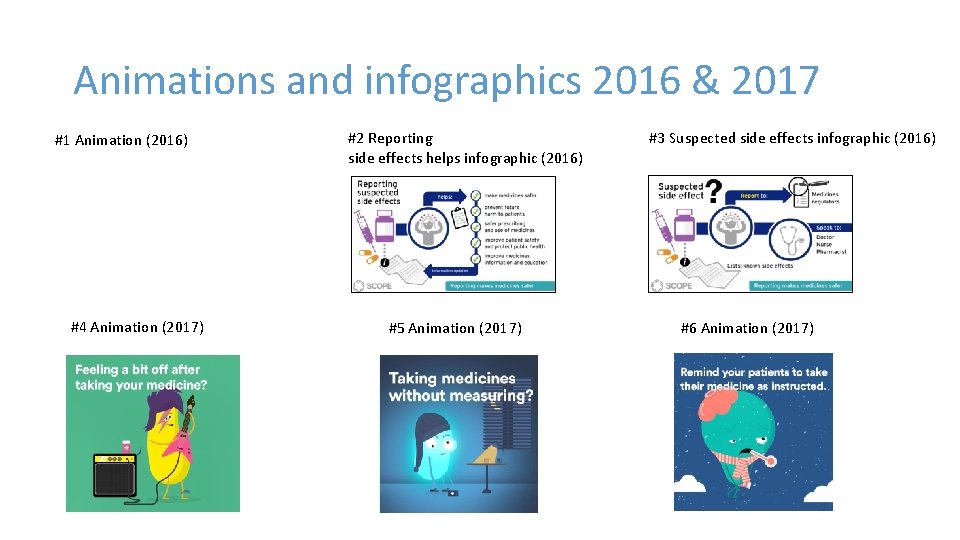
Animations and infographics 2016 & 2017 #1 Animation (2016) #4 Animation (2017) #2 Reporting side effects helps infographic (2016) #5 Animation (2017) #3 Suspected side effects infographic (2016) #6 Animation (2017)

New animations 2018 #7 Animation (2018) #8 Animation (2018) #10 Image of three characters used for teaser (2018) #9 Animation (2018)

Launch and running order: 19 -23 November

Day 0: Friday, 16 November • Retweet post: “It’s #medsafetyweek next week! 28 medicines regulators around the globe join forces to encourage reporting side effects to medicines in children and pregnancy. Watch out for our tweets, join the conversation, and help us spread the word! #World. Childrens. Day @UNICEF”

Day 1: Monday, 19 November Tweet (please tweet these posts as many times during the day Released with … as appropriate and in the sequence you prefer) Audience “#medsafetyweek starts today! 28 medicines regulators around Asset 10: image of three the globe join forces to encourage reporting side effects to characters (will be provided by medicines in children and pregnancy. Watch out for our tweets, UMC). join the conversation, and help us spread the word!” All (Public, patients and HCPs) “Did you know medicines may affect your baby and children in Asset 7: Children are not mini different ways? Sometimes medicines can pass to a baby during adults (2018) pregnancy or breastfeeding. Reporting side effects helps the safe use of medicines for babies and children. #medsafetyweek” Parents, carers “Reporting suspected side effects improves medicines safety for everyone. Watch our video to find out more. #medsafetyweek” Asset 1: Animation (2016) (also All (Public, hosted on You. Tube but uploading patients and direct to Twitter will autoplay) HCPs) “Reporting side effects can lead to new warnings for patients, making medicines safer for everyone. See how reporting helps us all stay healthy. #medsafetyweek” Asset 2: Reporting side effects helps infographic (2016) All (Public, patients and HCPs)

Day 2: Tuesday, 20 November Universal Children’s day Tweet (please tweet these posts as many times during the day as Released with … appropriate and in the sequence you prefer) Audience “Make sure you’re giving your baby or child the right medicine in the right dose, using the right spoon, syringe or device. Reporting side effects helps the safe use of medicines for babies and children #medsafetyweek #World. Childrens. Day @UNICEF (link to reporting system)” Parents, carers Asset 7: Children are not mini adults (2018) “Did you know that medicines affect your baby and children in Asset 8: Pregnant or different ways? breastfeeding? (2018) Medicines taken when pregnant or breastfeeding means they may reach your baby. Always speak to a doctor. Reporting side effects helps the safe use of medicines for babies and children #medsafetyweek #World. Childrens. Day @UNICEF” Mothers, expectant mothers “Make sure the medicine is right for the parent, baby or child. Discuss the potential of side effects and the importance of following recommended dosages and instruction. Reporting side effects helps the safe use of medicines for babies and children #medsafetyweek #World. Childrens. Day @UNICEF” HCPs Asset 9: Is the medicine right for your patient (2018)

Day 3: Wednesday, 21 November Tweet (please tweet these posts as many times during the day Released with … as appropriate and in the sequence you prefer) Audience “You know your baby or child best. If you suspect they're experiencing a side effect after taking medicine, let us know: link to reporting system). Reporting side effects helps the safe use of medicines for babies and children. #medsafetyweek” Asset 7: Children are not mini adults (2018) Parents, carers “Taken a medicine and feel unwell? You might be experiencing a side effect - make sure you report it to us so we can make medicines safer for everyone #medsafetyweek (link to reporting system)” Asset 4: Feeling a bit off (2017) Public, patients “Reporting side effects can lead to new warnings for patients. #medsafetyweek See the positive impact of reporting: [link to full animation on YT]” Asset 2: Reporting side effects helps infographic (2016) All (Public, patients and HCPs) “Thinking about having a baby or are pregnant? Speak to your doctor about taking medicines, as you may need to change. Sometimes side effects may appear many years later. Reporting side effects helps the safe use of medicines for babies and children. #medsafetyweek” Asset 8: Pregnant or breastfeeding? (2018) Mothers, expectant mothers

Day 4: Thursday, 22 November – HCP focus Tweet (please tweet these posts as many times during the day as appropriate and in the sequence you prefer) Released with … Audience “Make sure the medicine is right for the parent, baby or child. Asset 9: Is the medicine right for your HCPs Discuss the potential of side effects and the importance patient (2018) of following recommended dosages and instruction. Reporting side effects helps the safe use of medicines for babies and children #medsafetyweek” “Always discuss potential side effects with patients when you Asset 6: Remind your patients (2017) give medicines. Report side effects to us! #medsafetyweek (link to reporting system)” “Do your patients know how to report a suspected side effect? Reporting side effects helps improve the safety of medicines for everyone #medsafetyweek” HCPs Asset 9: Is the medicine right for your HCPs patient (2018)

Day 5: Friday, 23 November Tweet (please tweet these posts as many times during the day as Released with … appropriate and in the sequence you prefer) Audience “Make sure you’re giving your baby or child the right medicine Asset 7: Children are not mini in the right dose, using the right spoon, syringe or device. adults (2018) Reporting side effects helps the safe use of medicines for babies and children #medsafetyweek #World. Childrens. Day (link to Yellow Card reporting system)” Parents, carers “Did you know that medicines affect your baby and children in Asset 8: Pregnant or different ways? breastfeeding? (2018) Medicines taken when pregnant or breastfeeding means they may reach your baby. Always speak to a doctor. Reporting side effects helps the safe use of medicines for babies and children #medsafetyweek” Mothers, expectant mothers “Make sure the medicine is right for the parent, baby or child. Discuss the potential of side effects and the importance of following recommended dosages and instruction. Reporting side effects helps the safe use of medicines for babies and children #medsafetyweek” HCPs Asset 9: Is the medicine right for your patient (2018)

Regulators supporting the campaign 1. Belgium 13. Ireland 25. UK 2. Bulgaria 14. Italy 26. Australia 3. Croatia 15. Latvia 27. Mexico 4. Czech Republic 16. Lithuania 28. New Zealand 5. Denmark 17. Malta 6. Estonia 18. Netherlands 7. France 19. Poland 8. Finland 20. Portugal 9. Greece 21. Romania 10. Germany 22. Slovak Republic 11. Hungary 23. Slovenia 12. Iceland 24. Sweden Supporting • EC • EMA • EURORDIS

Thank you for supporting this important public health campaign
 Azalastyna
Azalastyna Adr awareness
Adr awareness Adverse reaction definition
Adverse reaction definition Adverse reaction definition
Adverse reaction definition Adverse reaction definition
Adverse reaction definition Cvs privacy awareness and hipaa training answers
Cvs privacy awareness and hipaa training answers Adap certificate
Adap certificate Example of substitution with exhausted drug is
Example of substitution with exhausted drug is Neurosurgery
Neurosurgery Adr tabela
Adr tabela National dangerous goods training consortium
National dangerous goods training consortium Adr shopping
Adr shopping Adr
Adr Istisnai miktar muafiyeti
Istisnai miktar muafiyeti Sdr adr
Sdr adr Säiliötyö koulutus
Säiliötyö koulutus During _______ branching, only car is updated with adr
During _______ branching, only car is updated with adr Podlimitná preprava adr
Podlimitná preprava adr































