Terapie Cellulari Immunomodulazione e Medicina Rigenerativa per la






![Hb. A 1 c [%] Diabetes Care 2016; 39: 1230 -1240 Hb. A 1 c [%] Diabetes Care 2016; 39: 1230 -1240](https://slidetodoc.com/presentation_image/59297dfe72306c7b150e881d84fa496d/image-12.jpg)






- Slides: 25

Terapie Cellulari, Immunomodulazione e Medicina Rigenerativa per la Cura del Diabete Camillo Ricordi, MD, FNAI Director, Diabetes Research Institute and Cell Transplant Center University of Miami Chairperson, NIH CIT SC Fellow, National Academy of Inventors ricordi@miami. edu


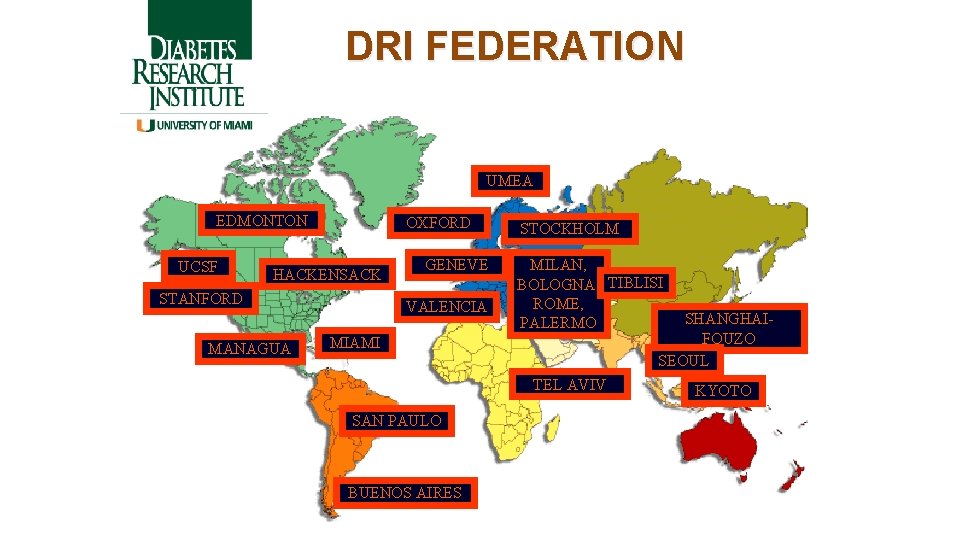
DRI FEDERATION UMEA EDMONTON UCSF OXFORD HACKENSACK STANFORD MANAGUA GENEVE VALENCIA STOCKHOLM MILAN, BOLOGNA, TIBLISI ROME, PALERMO SHANGHAIFOUZO SEOUL MIAMI TEL AVIV SAN PAULO BUENOS AIRES KYOTO

1988 – 2018 30 Years of the Ricordi Chamber (Diabetes, 1988)


1991

TRANSPLANTATION OF INSULIN PRODUCING CELLS FOR TREATMENT OF DIABETES

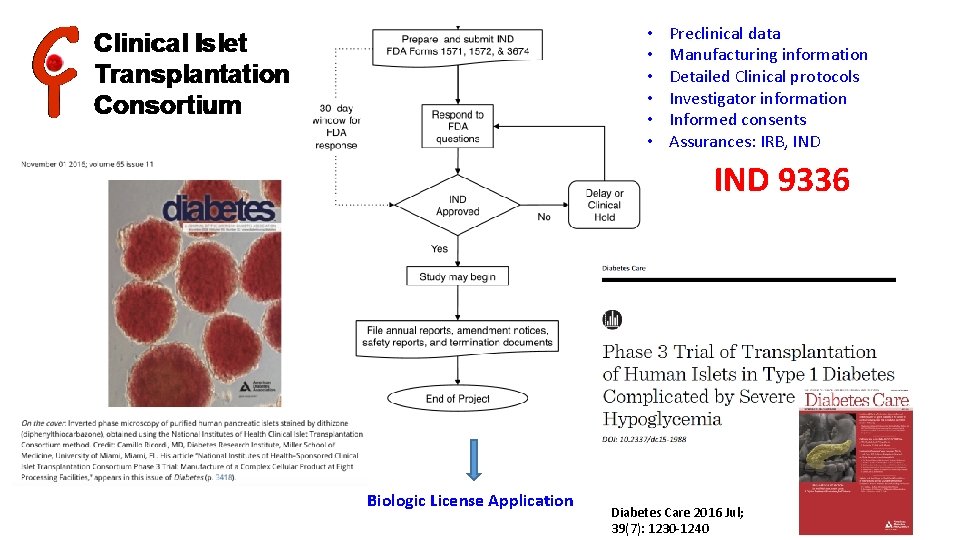
• • • Preclinical data Manufacturing information Detailed Clinical protocols Investigator information Informed consents Assurances: IRB, IND 9336 Biologic License Application Diabetes Care 2016 Jul; 39(7): 1230 -1240

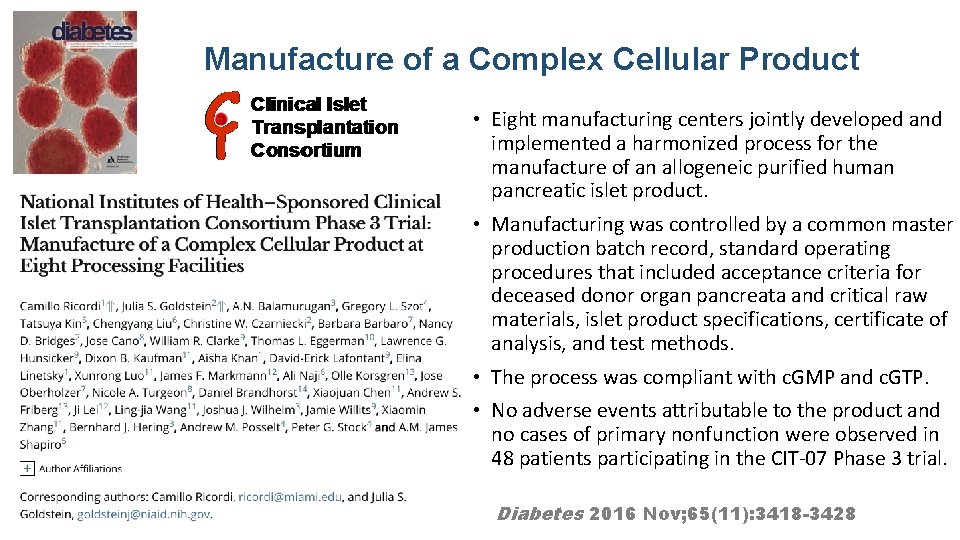
Manufacture of a Complex Cellular Product • Eight manufacturing centers jointly developed and implemented a harmonized process for the manufacture of an allogeneic purified human pancreatic islet product. • Manufacturing was controlled by a common master production batch record, standard operating procedures that included acceptance criteria for deceased donor organ pancreata and critical raw materials, islet product specifications, certificate of analysis, and test methods. • The process was compliant with c. GMP and c. GTP. • No adverse events attributable to the product and no cases of primary nonfunction were observed in 48 patients participating in the CIT-07 Phase 3 trial. Diabetes 2016 Nov; 65(11): 3418 -3428

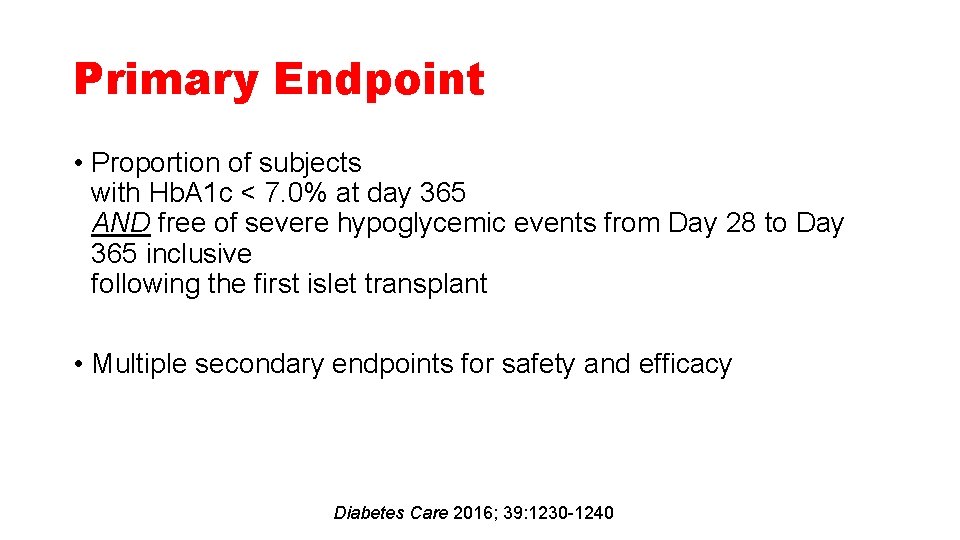
Primary Endpoint • Proportion of subjects with Hb. A 1 c < 7. 0% at day 365 AND free of severe hypoglycemic events from Day 28 to Day 365 inclusive following the first islet transplant • Multiple secondary endpoints for safety and efficacy Diabetes Care 2016; 39: 1230 -1240

Primary Endpoint Diabetes Care 2016; 39: 1230 -1240
![Hb A 1 c Diabetes Care 2016 39 1230 1240 Hb. A 1 c [%] Diabetes Care 2016; 39: 1230 -1240](https://slidetodoc.com/presentation_image/59297dfe72306c7b150e881d84fa496d/image-12.jpg)
Hb. A 1 c [%] Diabetes Care 2016; 39: 1230 -1240

% of Patients with SHE Diabetes Care 2016; 39: 1230 -1240

Islet Allograft Survival L ONS A I NT ATI E ERV D I NF OBS CO HED LIS B U P UN

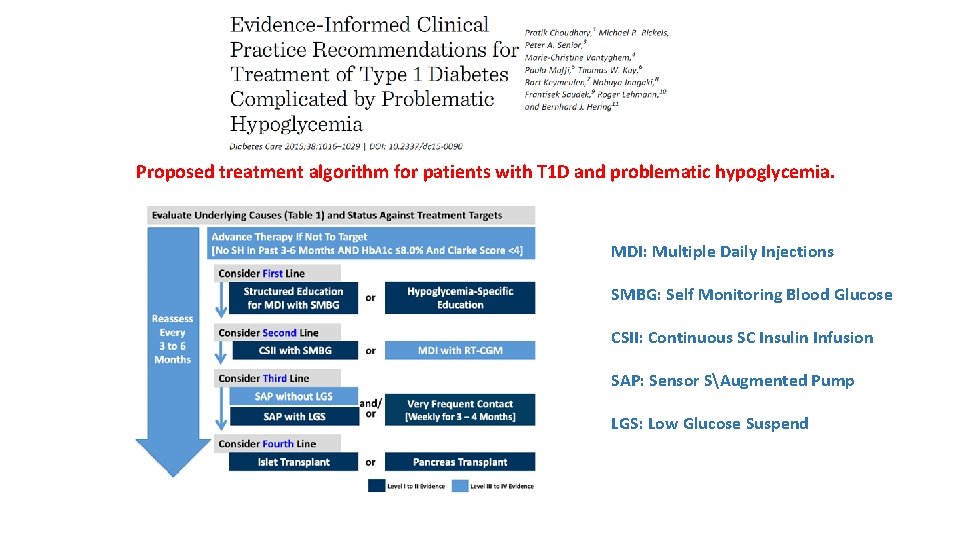
Proposed treatment algorithm for patients with T 1 D and problematic hypoglycemia. MDI: Multiple Daily Injections SMBG: Self Monitoring Blood Glucose CSII: Continuous SC Insulin Infusion SAP: Sensor SAugmented Pump LGS: Low Glucose Suspend

Outcomes for Adults with Type 1 Diabetes Referred with Severe Hypoglycaemia and/or Referred for Islet Transplantation to a Specialist Hypoglycaemia Service M. L. Byrne, D. Hopkins, W. Littlejohn, R. Beckford, P. Srinivasan, N. Heaton, S. A. Amiel, P. Choudhary King’s College London 47. 2% of patients presenting with problematic hypoglycaemia resolved with optimal medical therapy, with a further 25% achieving clinically relevant improvement, however 27. 8% required transplantation despite access to all therapies. Horm Metab Res 2015; 47 (01): 9 -15

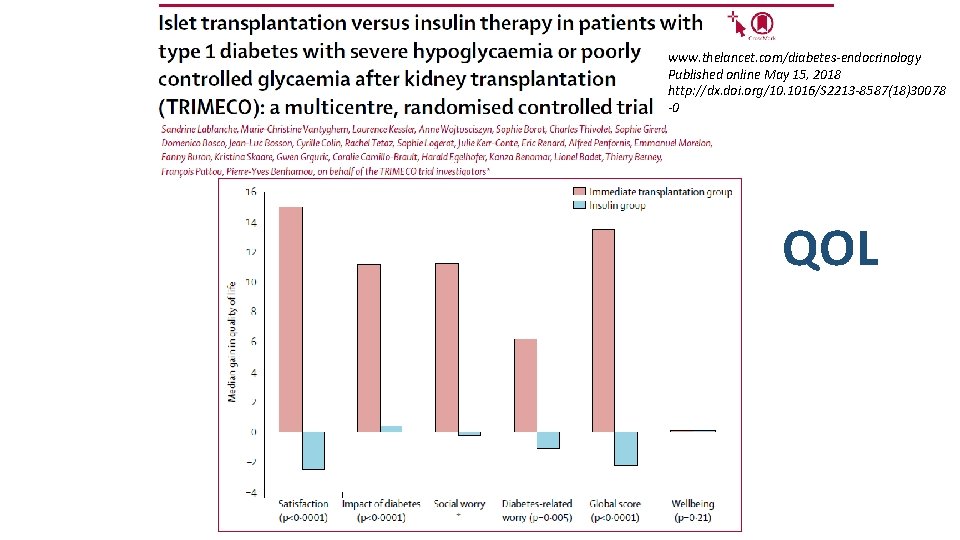
www. thelancet. com/diabetes-endocrinology Published online May 15, 2018 http: //dx. doi. org/10. 1016/S 2213 -8587(18)30078 -0 QOL

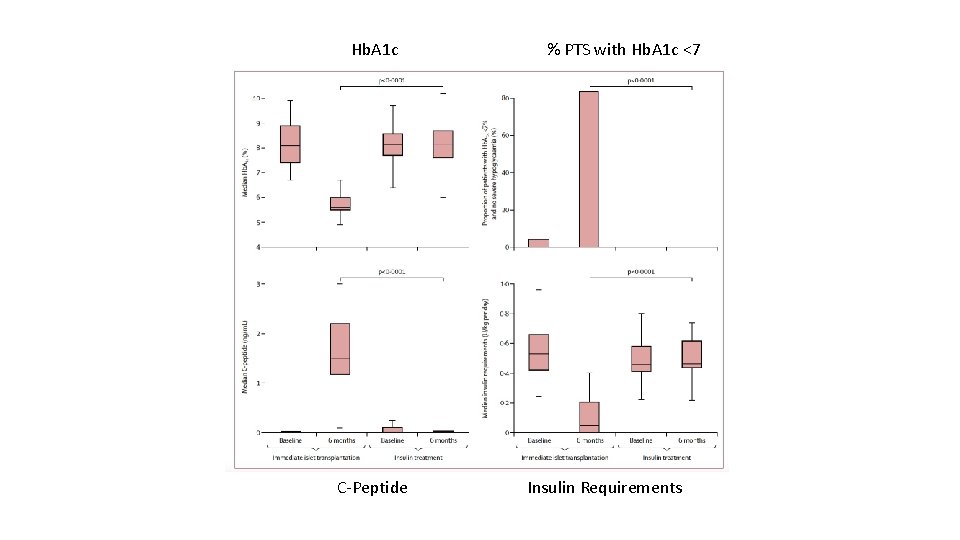
Hb. A 1 c C-Peptide % PTS with Hb. A 1 c <7 Insulin Requirements

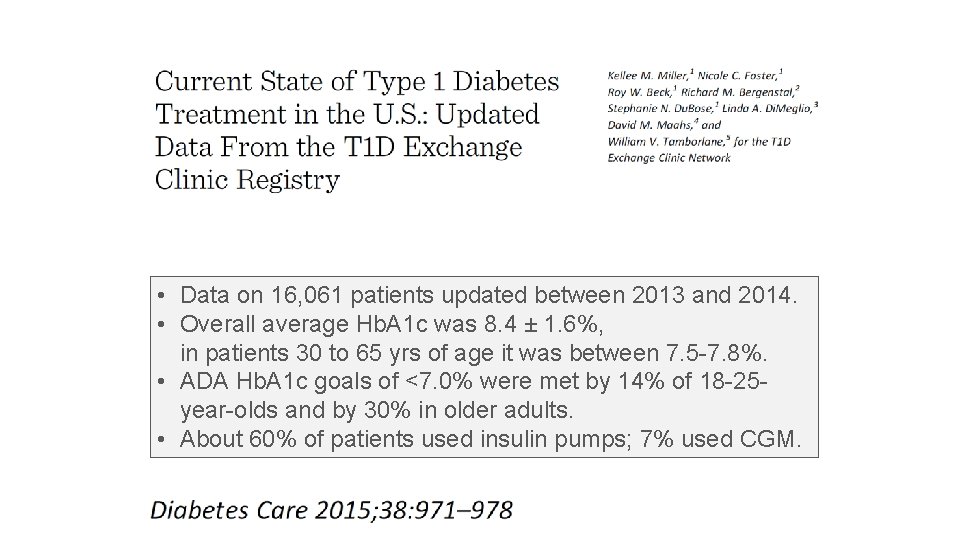
• Data on 16, 061 patients updated between 2013 and 2014. • Overall average Hb. A 1 c was 8. 4 ± 1. 6%, in patients 30 to 65 yrs of age it was between 7. 5 -7. 8%. • ADA Hb. A 1 c goals of <7. 0% were met by 14% of 18 -25 year-olds and by 30% in older adults. • About 60% of patients used insulin pumps; 7% used CGM.


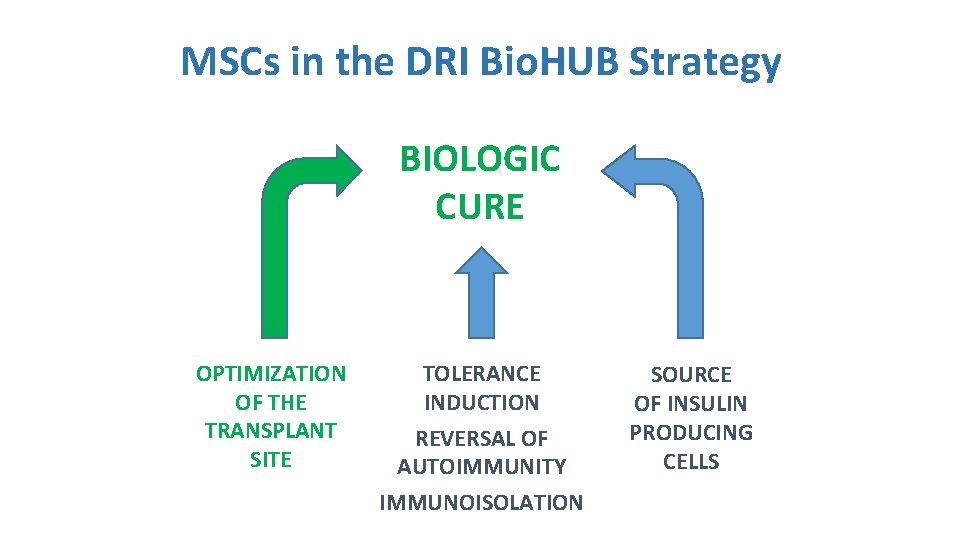
MSCs in the DRI Bio. HUB Strategy BIOLOGIC CURE OPTIMIZATION OF THE TRANSPLANT SITE TOLERANCE INDUCTION REVERSAL OF AUTOIMMUNITY IMMUNOISOLATION SOURCE OF INSULIN PRODUCING CELLS



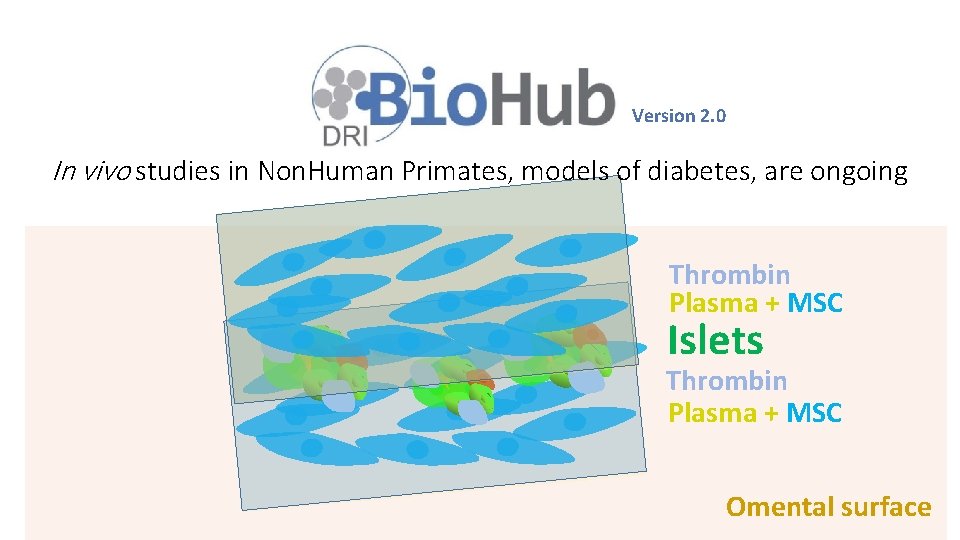
Bioengineering of an Intraabdominal Endocrine Pancreas

Version 2. 0 In vivo studies in Non. Human Primates, models of diabetes, are ongoing Thrombin Plasma + MSC Islets Thrombin Plasma + MSC Omental surface
 Etapele terapiei logopedice
Etapele terapiei logopedice Elektrokonvulzivní terapie zkušenosti
Elektrokonvulzivní terapie zkušenosti Biocatalizzatori cellulari
Biocatalizzatori cellulari Cellulari 3
Cellulari 3 Organuli cellulari
Organuli cellulari Dimensioni organuli cellulari
Dimensioni organuli cellulari Cellula animale zanichelli
Cellula animale zanichelli Quante volte tocchiamo il cellulare
Quante volte tocchiamo il cellulare Giunzioni comunicanti
Giunzioni comunicanti Studenti e professori uniti per
Studenti e professori uniti per Catullus 84
Catullus 84 Kruta ir kruta braska ir braska
Kruta ir kruta braska ir braska Multas per gentes et multa per aequora vectus
Multas per gentes et multa per aequora vectus Moltiplicazioni con numeri periodici
Moltiplicazioni con numeri periodici 1 hour 60 minutes
1 hour 60 minutes 27 miles per gallon into kilometers per liter
27 miles per gallon into kilometers per liter Longum iter est
Longum iter est Coop per me e per te
Coop per me e per te Siti pergi selama 360 menit + 120 menit berapa jam kah itu
Siti pergi selama 360 menit + 120 menit berapa jam kah itu Per capita vs per stirpes
Per capita vs per stirpes Il mio diletto canto
Il mio diletto canto Per stirpes v per capita
Per stirpes v per capita Docimasia gastrointestinal de breslau
Docimasia gastrointestinal de breslau Coni medicina dello sport
Coni medicina dello sport Rcu medicina
Rcu medicina Sindrome postneumonectomia
Sindrome postneumonectomia