Synthesis of Eicosanoids Glycerolipids and Isoprenoids Eicosanoids Eicosanoids







- Slides: 23

Synthesis of Eicosanoids, Glycerolipids and Isoprenoids

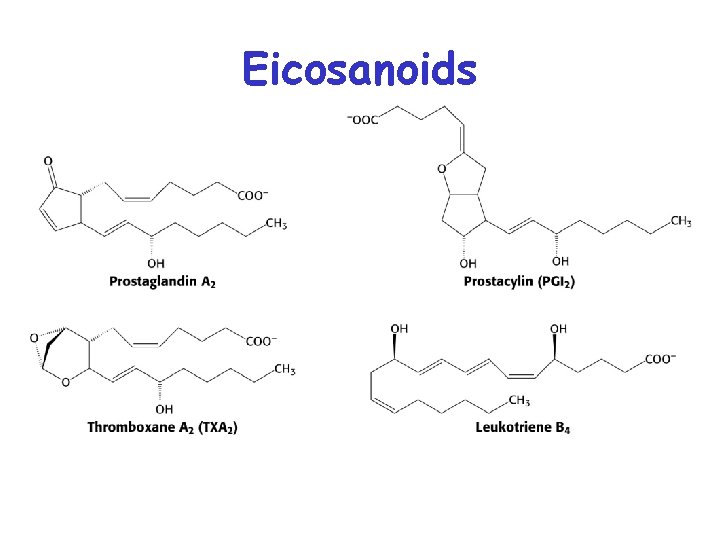
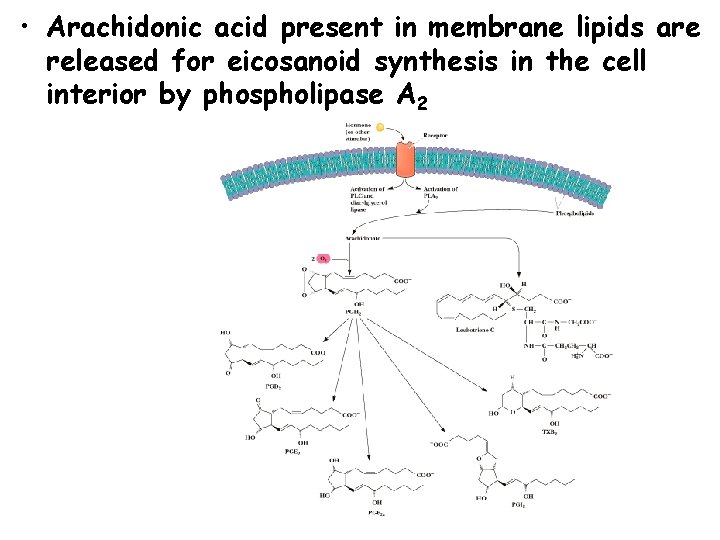
Eicosanoids • Eicosanoids are important regulatory molecules • Referred to as local regulators. Function where they are produced. • Two classes: Prostaglandins/thromboxanes, and Leukotrienes • Prostaglandins – mediate pains sensitivity, inflammation and swelling • Thromboxanes – involved in blood clotting, constriction of arteries • Leukotrienes – attract white cells, involved inflammatory diseases (asthma, arthritis, etc. . )

Eicosanoids

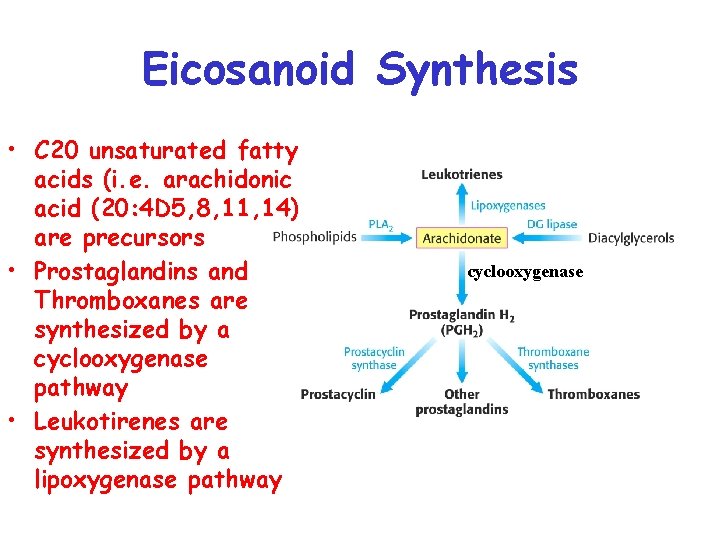
Eicosanoid Synthesis • C 20 unsaturated fatty acids (i. e. arachidonic acid (20: 4 D 5, 8, 11, 14) are precursors • Prostaglandins and Thromboxanes are synthesized by a cyclooxygenase pathway • Leukotirenes are synthesized by a lipoxygenase pathway cyclooxygenase

• Arachidonic acid present in membrane lipids are released for eicosanoid synthesis in the cell interior by phospholipase A 2

Cyclooxygenase (COX) Inhibitors • Two COX isozymes: COX-1 and COX-2. • COX-1 – important in regulating mucin secretion in stomach • COX-2 – promotes pain and inflammation and fever (involved in prostaglandin synthesis). • Asprin (acetylsalicylate) non-specific COX inhibitor. Acts by acetylating an essential serine residue in the active site. • Because asprin inhibits COX-1, causes stomach upset and other side effects. • New drugs (Vioxx and Celebrex) specifically inhibit COX-2


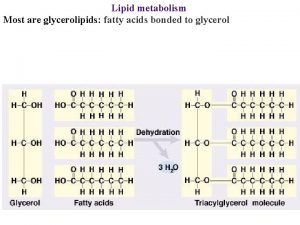
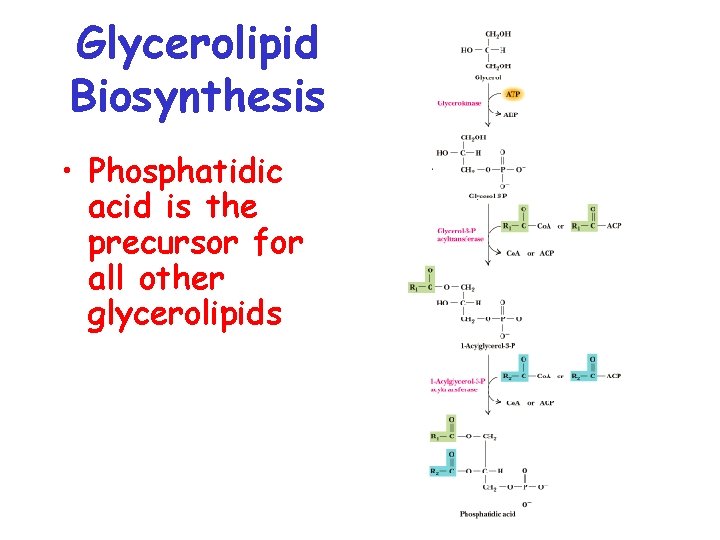
Glycerolipid Biosynthesis • Important for the synthesis of membrane lipids and triacylglycerol • Synthesis occurs primarily in ER • Phosphatidic acid (PA) is the precursor for all other glycerolipids in eukaryotes • PA is made either into diacylglycerol (DAG) or CDP-DAG

Glycerolipid Biosynthesis • Phosphatidic acid is the precursor for all other glycerolipids



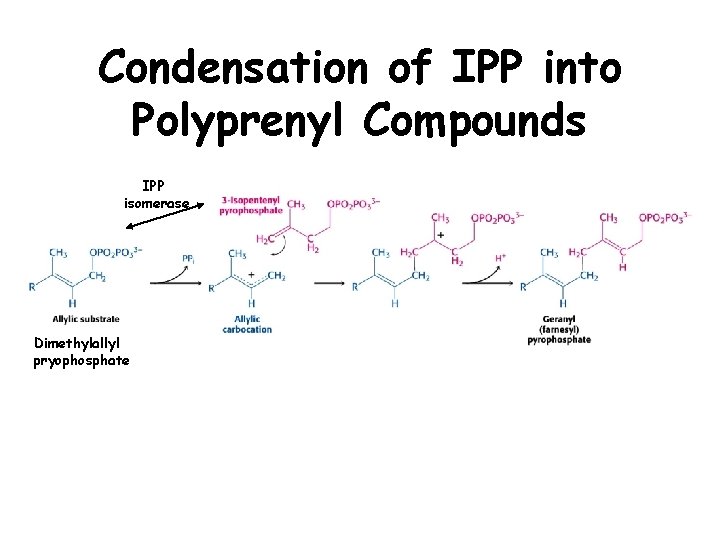
Isoprenoid Synthesis • Involves formation of isopentenyl pyrophosphate (IPP) monmers. • IPP is conjugated in a head to tail manner to generate polyprenyl compounds.

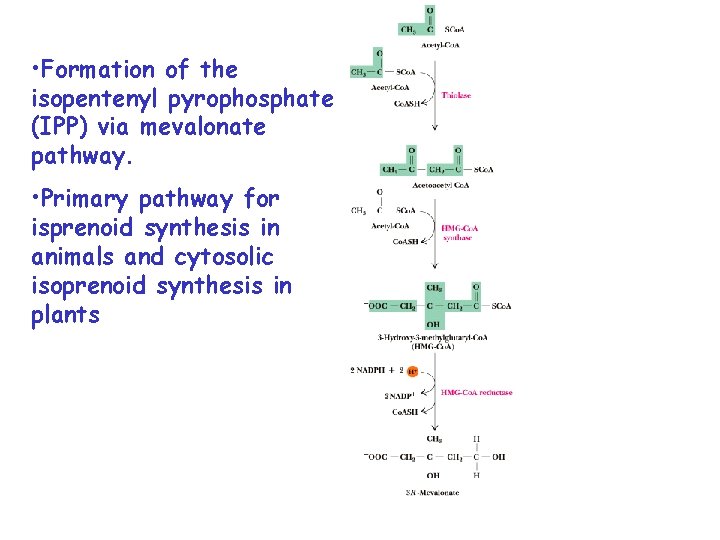
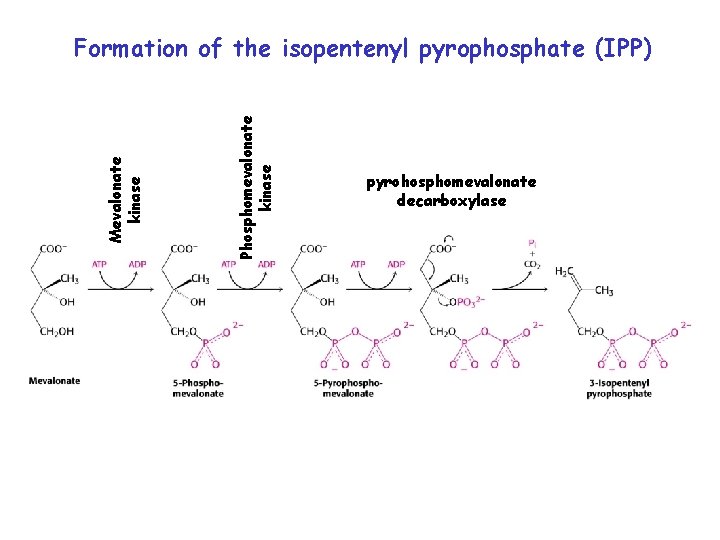
• Formation of the isopentenyl pyrophosphate (IPP) via mevalonate pathway. • Primary pathway for isprenoid synthesis in animals and cytosolic isoprenoid synthesis in plants

Phosphomevalonate kinase Mevalonate kinase Formation of the isopentenyl pyrophosphate (IPP) pyrohosphomevalonate decarboxylase

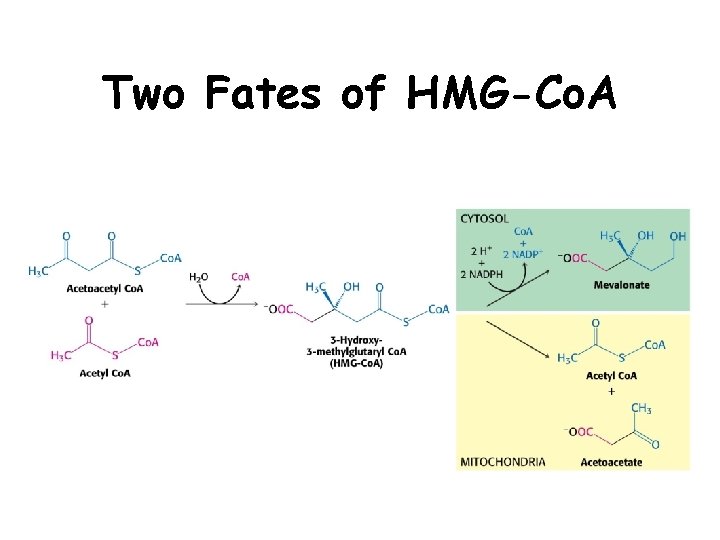
Two Fates of HMG-Co. A

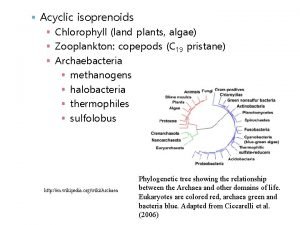
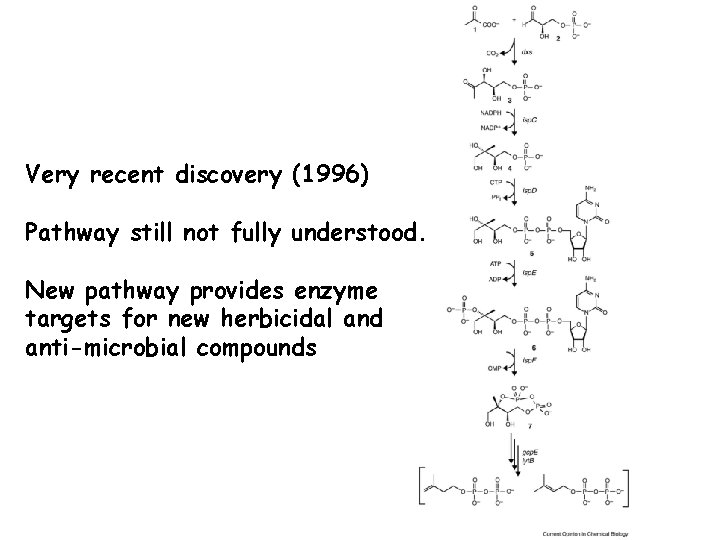
Bacteria and Plants Synthesize IPP via Non-Mevalonate Pathway • In plants and most bacteria, IPP is synthesized from the condensation of glyceraldehyde-3 phosphate (3 carbons) and pyruvate (3 carbons). • Forms a 5 carbon intermediate through transketolase type reaction (transfer of 2 carbon aldehyde from pyruvate to G-3 -P). • Occurs in chloroplast of plants. Involved in synthesis of chlorophyll, carotenoids, Vitamins A, E and K.

Very recent discovery (1996) Pathway still not fully understood. New pathway provides enzyme targets for new herbicidal and anti-microbial compounds

Condensation of IPP into Polyprenyl Compounds IPP isomerase Dimethylallyl pryophosphate

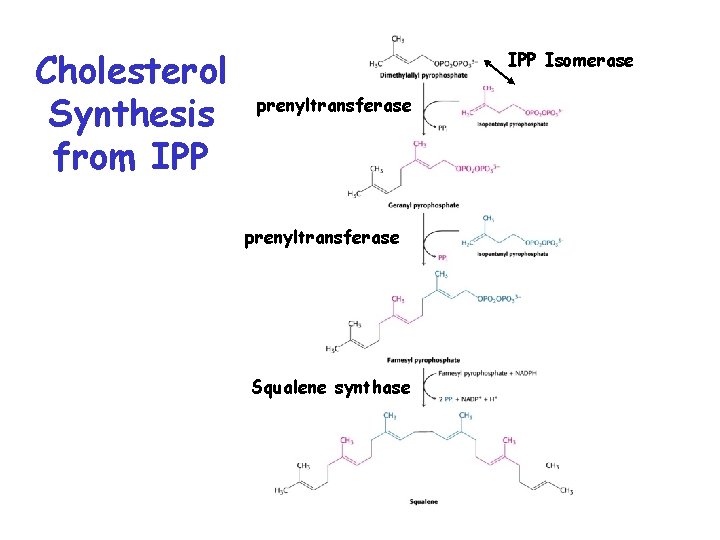
Cholesterol Synthesis from IPP Isomerase prenyltransferase Squalene synthase

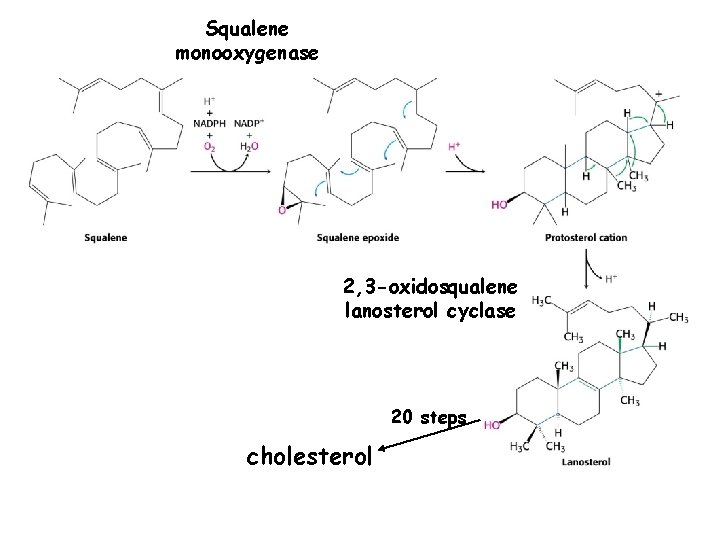
Squalene monooxygenase 2, 3 -oxidosqualene lanosterol cyclase 20 steps cholesterol

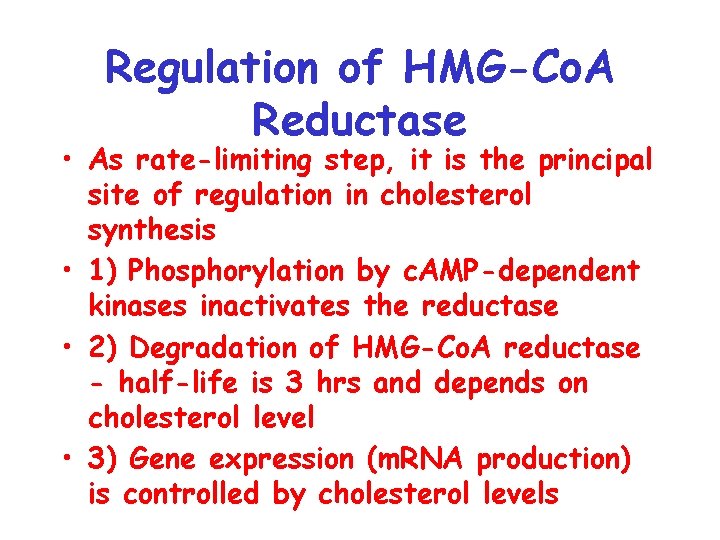
Regulation of HMG-Co. A Reductase • As rate-limiting step, it is the principal site of regulation in cholesterol synthesis • 1) Phosphorylation by c. AMP-dependent kinases inactivates the reductase • 2) Degradation of HMG-Co. A reductase - half-life is 3 hrs and depends on cholesterol level • 3) Gene expression (m. RNA production) is controlled by cholesterol levels

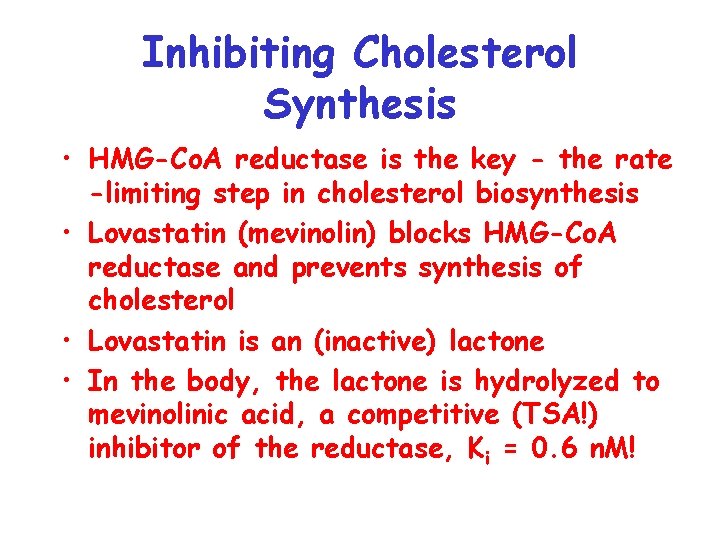
Inhibiting Cholesterol Synthesis • HMG-Co. A reductase is the key - the rate -limiting step in cholesterol biosynthesis • Lovastatin (mevinolin) blocks HMG-Co. A reductase and prevents synthesis of cholesterol • Lovastatin is an (inactive) lactone • In the body, the lactone is hydrolyzed to mevinolinic acid, a competitive (TSA!) inhibitor of the reductase, Ki = 0. 6 n. M!

 Function of eicosanoids
Function of eicosanoids Eicosanoid synthesis
Eicosanoid synthesis It is an integrated analysis and synthesis
It is an integrated analysis and synthesis Section 12-3 rna and protein synthesis answer key
Section 12-3 rna and protein synthesis answer key Apa yang dimaksud rangkuman
Apa yang dimaksud rangkuman Difference between linear and convergent synthesis
Difference between linear and convergent synthesis Reaction type: synthesis
Reaction type: synthesis Synthesis level of comprehension
Synthesis level of comprehension Hydrolysis and dehydration synthesis
Hydrolysis and dehydration synthesis Clorobutanol que es
Clorobutanol que es Missense mutation in sickle cell anemia
Missense mutation in sickle cell anemia Image quilting for texture synthesis and transfer
Image quilting for texture synthesis and transfer Protein synthesis and mutations
Protein synthesis and mutations Protein synthesis and mutations
Protein synthesis and mutations Verilog hdl: a guide to digital design and synthesis pdf
Verilog hdl: a guide to digital design and synthesis pdf Gravitation and newton's synthesis
Gravitation and newton's synthesis Integration and synthesis
Integration and synthesis Synthesis and sidedness of membranes
Synthesis and sidedness of membranes Rna and protein synthesis study guide
Rna and protein synthesis study guide Verilog hdl: a guide to digital design and synthesis
Verilog hdl: a guide to digital design and synthesis Diol formation from alkene
Diol formation from alkene Madhukar mittal
Madhukar mittal Synthesis and characterization of aspirin
Synthesis and characterization of aspirin Hydrolysis reaction
Hydrolysis reaction