Stoichiometry http www unit 5 orgchemistryStoichiometry html Stoichiometry







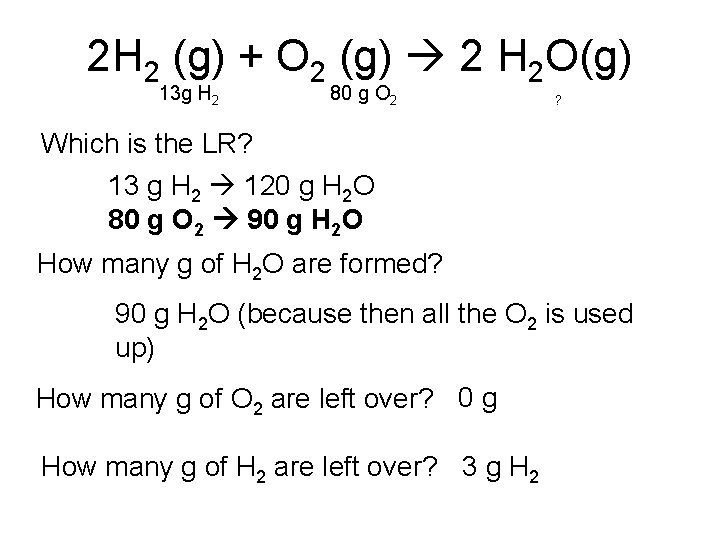
- Slides: 18

Stoichiometry http: //www. unit 5. org/chemistry/Stoichiometry. html

Stoichiometry • Stoichiometry – mass relationships between substances in a chemical reaction – based on the mole ratio – Involves finding amounts of reactants and products in a reaction • Mole Ratio – indicated by coefficients in a balanced equation 2 Mg + O 2 2 Mg. O Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

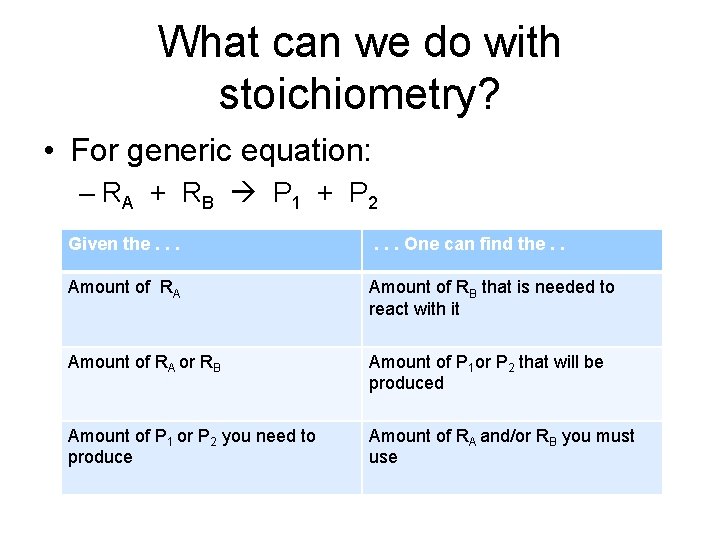
What can we do with stoichiometry? • For generic equation: – RA + RB P 1 + P 2 Given the. . . One can find the. . Amount of RA Amount of RB that is needed to react with it Amount of RA or RB Amount of P 1 or P 2 that will be produced Amount of P 1 or P 2 you need to produce Amount of RA and/or RB you must use

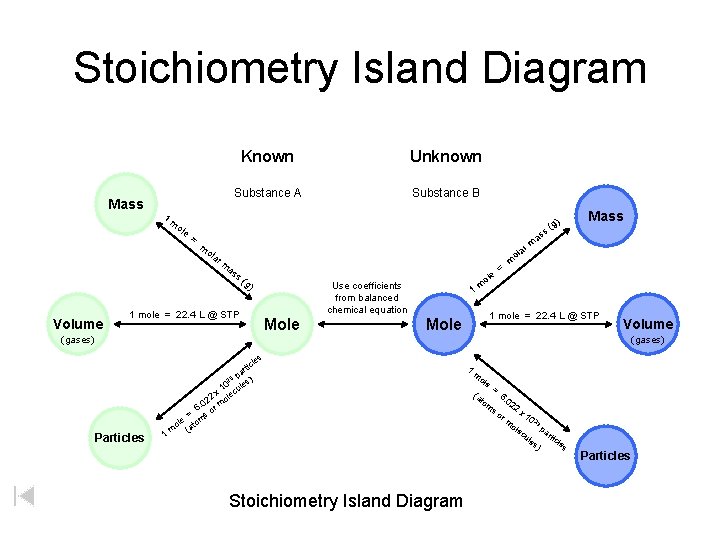
Stoichiometry Island Diagram Known Mass Unknown Substance A Substance B 1 m ole g) = m ola r m as s ( g Mass m = ) Volume a m ( ss 1 mole = 22. 4 L @ STP ole m 1 Use coefficients from balanced chemical equation Mole 1 mole = 22. 4 L @ STP Mole Volume (gases) Particles 1 les tic r pa ) 23 0 ules 1 x ec 22 mol 0. 6 or = ms ole to m (a Stoichiometry Island Diagram 1 m ole = (a to 6 m s o . 0 22 x 10 2 3 ole p ar cu les ticle ) s r m Particles

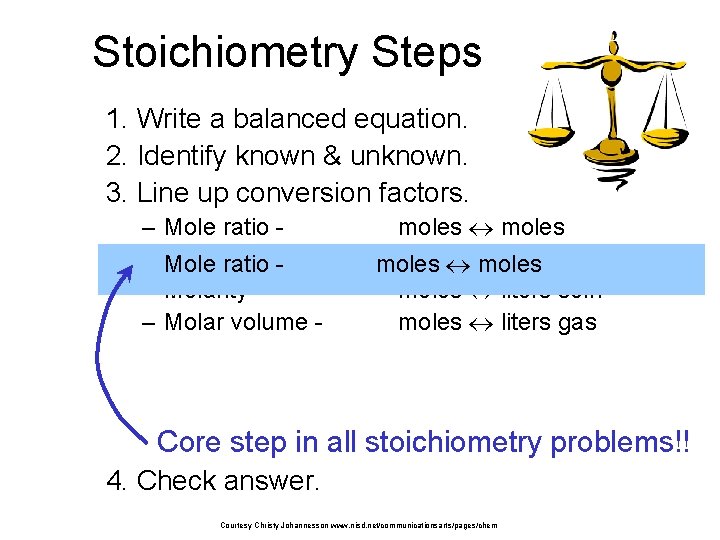
Stoichiometry Steps 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. – – – Mole ratio - Molar mass Mole ratio - Molarity - Molar volume - moles grams moles liters soln moles liters gas Core step in all stoichiometry problems!! 4. Check answer. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Stoichiometry 2 Ti. O 2 + 4 Cl 2 + 3 C 115 g x mol CO 2 + 2 CO + 2 Ti. Cl 4 4. 55 mol x molecules How many moles of chlorine will react with 4. 55 moles of carbon? x mol C = 4. 55 mol Cl 2 C 3 mol C 4 mol Cl 2 = 6. 07 mol Cl 2 How many grams of titanium (IV) oxide will react with 4. 55 moles of carbon? x g Ti. O 2 = 4. 55 mol C C 1 mol Ti. O 2 80 g Ti. O 2 2 mol Ti. O 2 3 mol C = 243 g Ti. O 2 How many molecules of Ti. Cl 4 will react with 115 g Ti. O 2? x molecules Ti. Cl 4 = 115 g Ti. O 2 1 mol Ti. O 2 2 mol Ti. Cl 4 6. 02 x 1023 molecules Ti. Cl 4 1 mol Ti. Cl 4 80 g Ti. O 2 2 mol Ti. O 2 = 8. 66 x 1023 molecules Ti. Cl 4 Ti. O 2 Ti. Cl 4

Island Diagram Helpful Reminders 1. Use coefficients from the equation only when crossing the middle bridge. The other six bridges always have “ 1 mol” before a substance’s formula. 2. The middle bridge conversion factor is the only one that has two different substances in it. The conversion factors for the other six bridges have the same substance in both the numerator and denominator. 3. The units on the islands at each end of the bridge being crossed appear in the top of the other conversion factor for that bridge.

Limiting Reactants Caution: this stuff is difficult to follow at first. Be patient.

Limiting Reactants • Limiting Reactant – used up in a reaction – determines the amount of product • Excess Reactant – added to ensure that the other reactant is completely used up – cheaper & easier to recycle Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

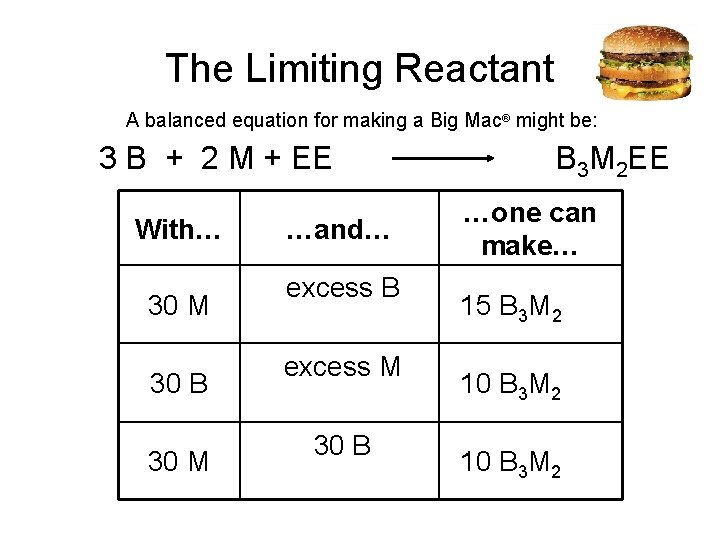
The Limiting Reactant A balanced equation for making a Big Mac® might be: 3 B + 2 M + EE B 3 M 2 EE With… …and… …one can make… 30 M excess B 15 B 3 M 2 30 B excess M 2 10 B 3 M 30 M 30 B 2 10 B 3 M

Limiting Reactants 1. Write a balanced chemical equation. 2. For each reactant, calculate the amount of product formed. 3. Smaller answer indicates: – limiting reactant – amount of product Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

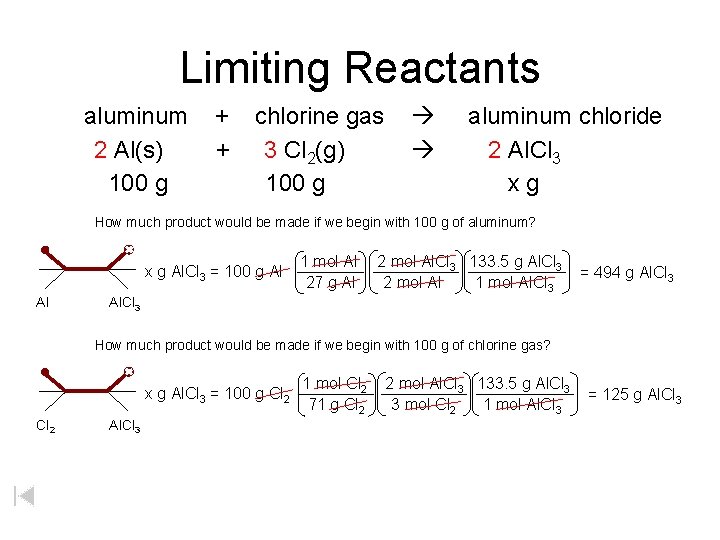
Limiting Reactants aluminum + chlorine gas aluminum chloride 2 Al(s) + 3 Cl 2(g) 2 Al. Cl 3 100 g x g How much product would be made if we begin with 100 g of aluminum? x g Al. Cl 3 = 100 g Al Al 1 mol Al 27 g Al 2 mol Al. Cl 3 133. 5 g Al. Cl 3 2 mol Al 1 mol Al. Cl 3 = 494 g Al. Cl 3 How much product would be made if we begin with 100 g of chlorine gas? x g Al. Cl 3 = 100 g Cl 2 Al. Cl 3 1 mol Cl 2 71 g Cl 2 2 mol Al. Cl 3 133. 5 g Al. Cl 3 3 mol Cl 2 1 mol Al. Cl 3 = 125 g Al. Cl 3

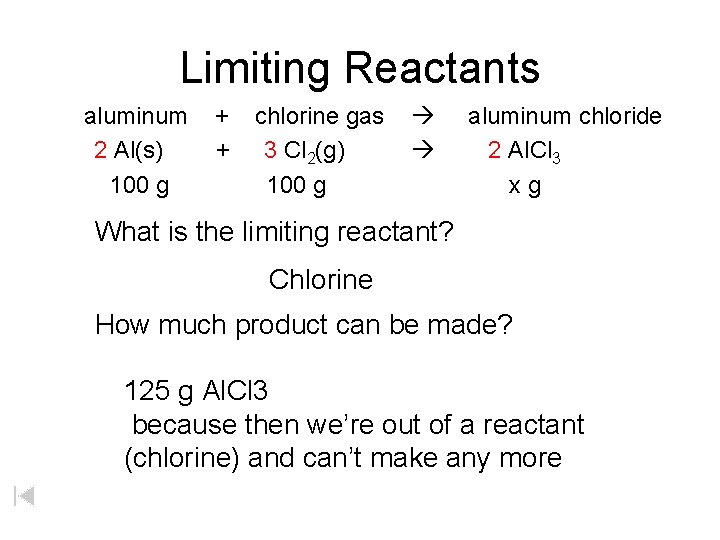
Limiting Reactants aluminum + chlorine gas aluminum chloride 2 Al(s) + 3 Cl 2(g) 2 Al. Cl 3 100 g x g What is the limiting reactant? Chlorine How much product can be made? 125 g Al. Cl 3 because then we’re out of a reactant (chlorine) and can’t make any more

2 Fe(s) + 3 Cl 2(g) 2 Fe. Cl 3(s) 223 g Fe 179 L Cl 2 ? Which is the limiting reactant? 223 g Fe 648 g Fe. Cl 3 179 L Cl 2 865 g Fe. Cl 3 How much Fe. Cl 3 can be formed? 648 g Fe. Cl 3

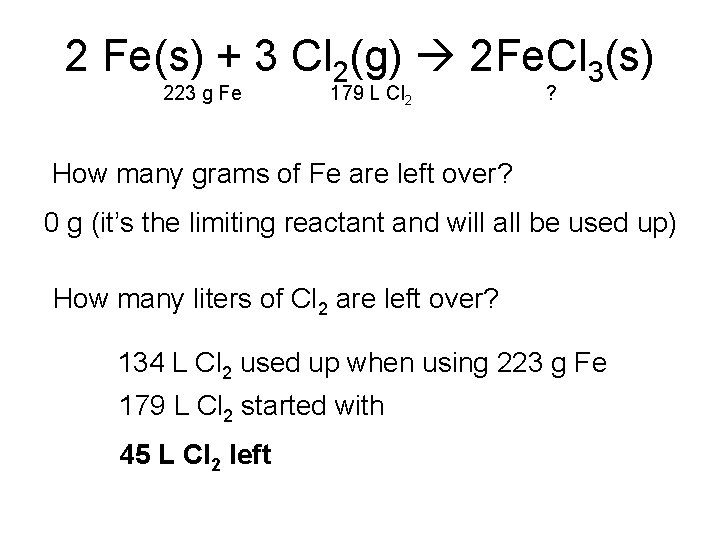
2 Fe(s) + 3 Cl 2(g) 2 Fe. Cl 3(s) 223 g Fe 179 L Cl 2 ? How many grams of Fe are left over? 0 g (it’s the limiting reactant and will all be used up) How many liters of Cl 2 are left over? 134 L Cl 2 used up when using 223 g Fe 179 L Cl 2 started with 45 L Cl 2 left
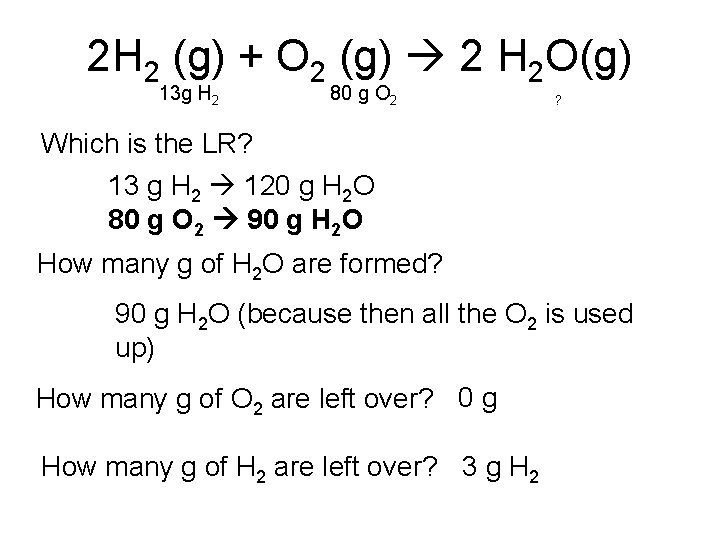
2 H 2 (g) + O 2 (g) 2 H 2 O(g) 13 g H 2 80 g O 2 ? Which is the LR? 13 g H 2 120 g H 2 O 80 g O 2 90 g H 2 O How many g of H 2 O are formed? 90 g H 2 O (because then all the O 2 is used up) How many g of O 2 are left over? 0 g How many g of H 2 are left over? 3 g H 2

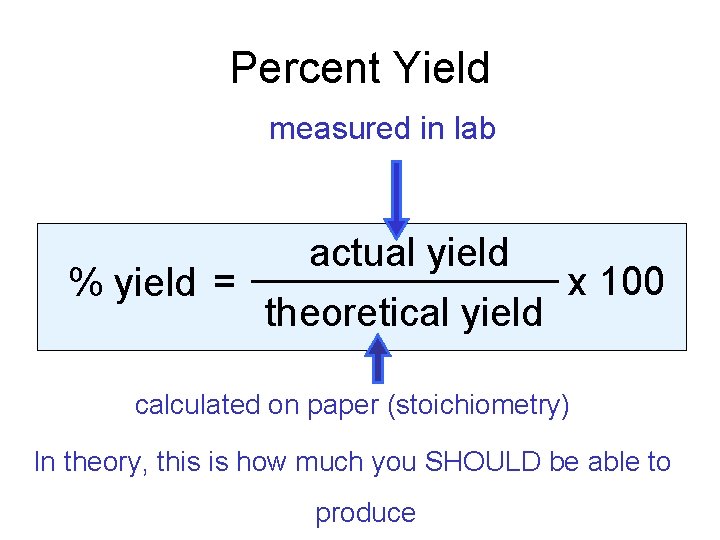
Percent Yield measured in lab % yield = actual yield theoretical yield x 100 calculated on paper (stoichiometry) In theory, this is how much you SHOULD be able to produce

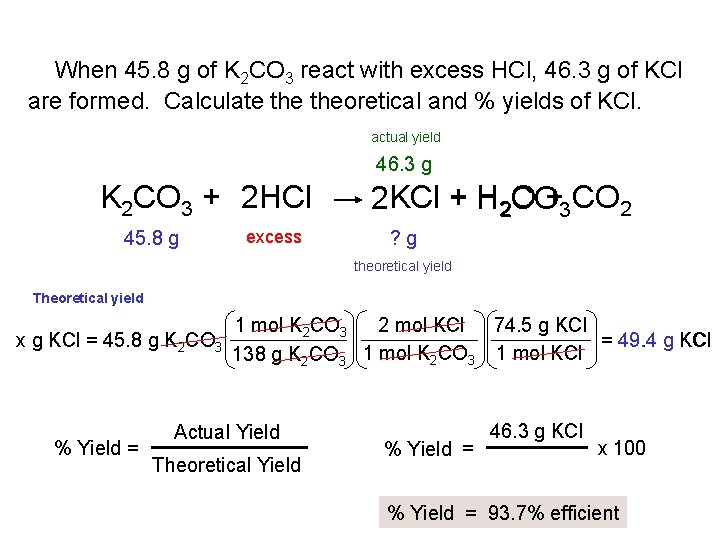
When 45. 8 g of K 2 CO 3 react with excess HCl, 46. 3 g of KCl are formed. Calculate theoretical and % yields of KCl. actual yield 46. 3 g K 2 CO 3 + 2 HCl 45. 8 g excess 2 KCl + H 2 O + CO CO 3 2 ? g theoretical yield Theoretical yield x g KCl = 45. 8 g K 2 CO 3 % Yield = 1 mol K 2 CO 3 2 mol KCl 74. 5 g KCl = 49. 4 g KCl 1 mol K CO 1 mol KCl 138 g K 2 CO 3 2 3 Actual Yield Theoretical Yield % Yield = 46. 3 g KCl x 100 % Yield = 93. 7% efficient