Stoichiometric relationships Where am I at Stations activity


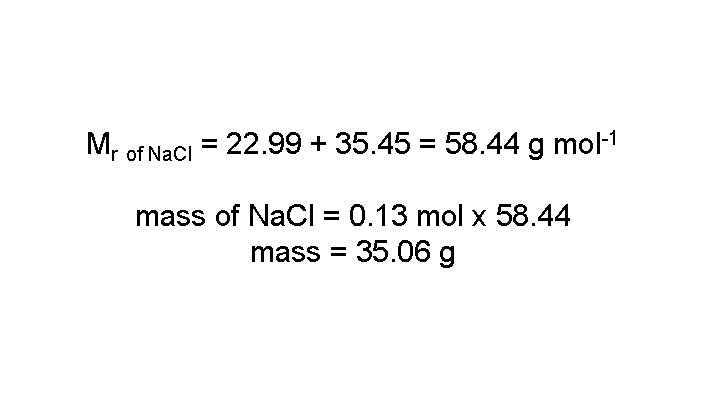






- Slides: 14

Stoichiometric relationships Where am I at? Stations activity

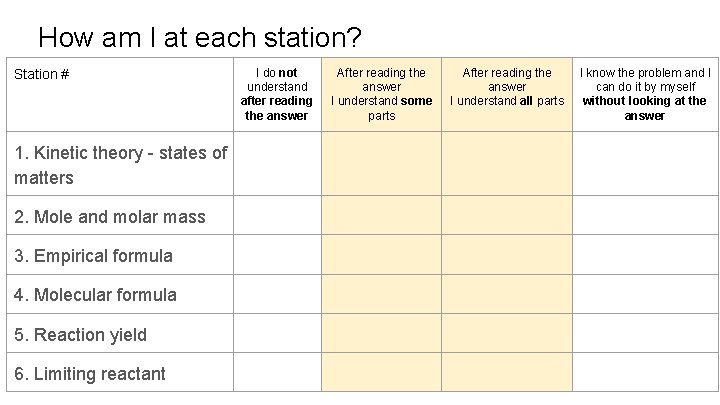
How am I at each station? Station # 1. Kinetic theory - states of matters 2. Mole and molar mass 3. Empirical formula 4. Molecular formula 5. Reaction yield 6. Limiting reactant I do not understand after reading the answer After reading the answer I understand some parts After reading the answer I understand all parts I know the problem and I can do it by myself without looking at the answer

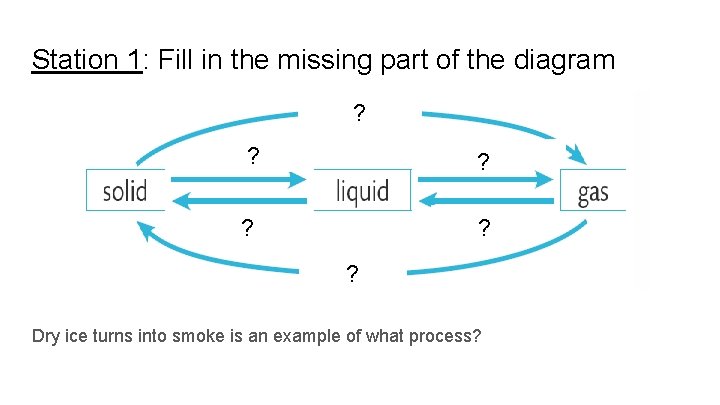
Station 1: Fill in the missing part of the diagram ? ? Dry ice turns into smoke is an example of what process?

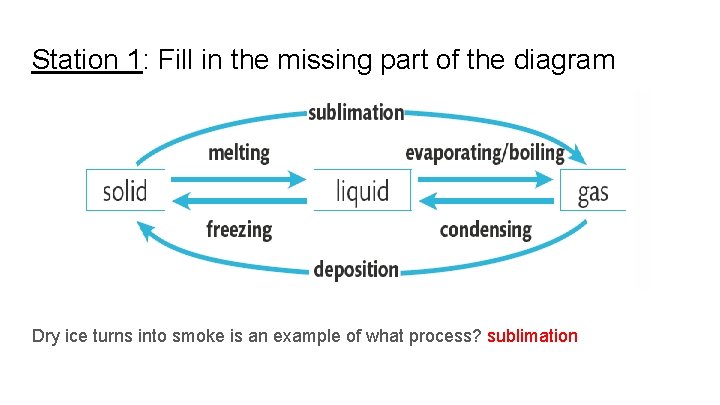
Station 1: Fill in the missing part of the diagram Dry ice turns into smoke is an example of what process? sublimation

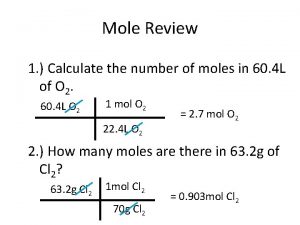
Station 2: Measure exactly 0. 13 moles of Na. Cl on an electric balance
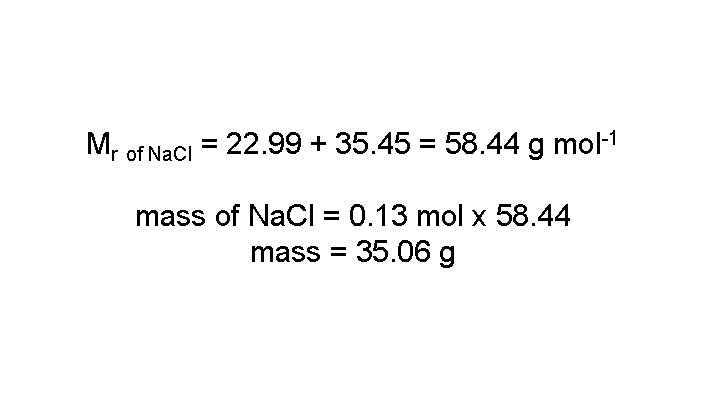
Mr of Na. Cl = 22. 99 + 35. 45 = 58. 44 g mol-1 mass of Na. Cl = 0. 13 mol x 58. 44 mass = 35. 06 g

Station 3:


Station 4: Calomel is a compound once used in the treatment of syphilis. It has the empirical formula Hg. Cl and a molar mass of 472. 08 g / mol. What is its molecular formula?


Station 5:


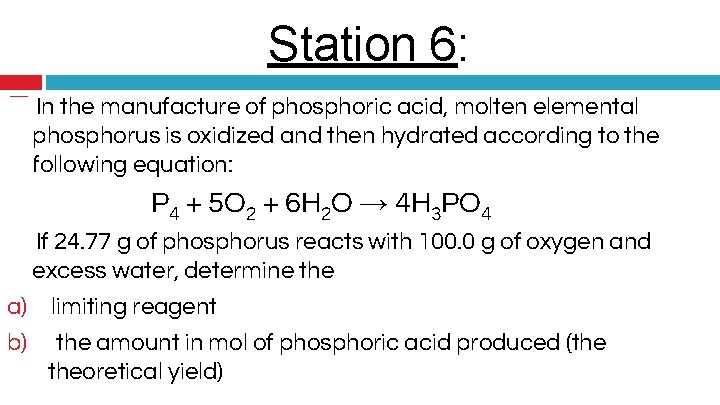
Station 6: � In the manufacture of phosphoric acid, molten elemental phosphorus is oxidized and then hydrated according to the following equation: P 4 + 5 O 2 + 6 H 2 O → 4 H 3 PO 4 � If 24. 77 g of phosphorus reacts with 100. 0 g of oxygen and excess water, determine the a) limiting reagent b) the amount in mol of phosphoric acid produced (the theoretical yield)

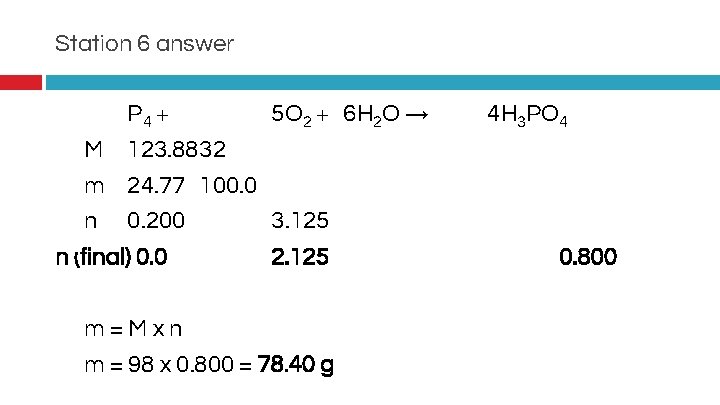
Station 6 answer P 4 + 5 O 2 + 6 H 2 O → � M 123. 8832 � m 24. 77 100. 0 � n 0. 200 3. 125 n (final) 0. 0 2. 125 �m=Mxn � m = 98 x 0. 800 = 78. 40 g 4 H 3 PO 4 0. 800
 Stoichiometry stations activity
Stoichiometry stations activity Mstockx
Mstockx Stoichiometric table for reversible reaction
Stoichiometric table for reversible reaction Molecular mass of octane
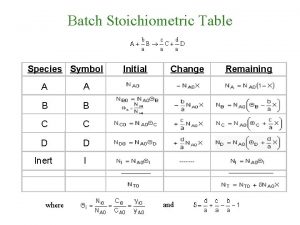
Molecular mass of octane Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric factor
Stoichiometric factor Stoichiometry table
Stoichiometry table Defining stoichiometry
Defining stoichiometry Stoichiometric gasoline
Stoichiometric gasoline Stoichiometry example
Stoichiometry example What is stoichiometric
What is stoichiometric Symbolaab
Symbolaab Stoichiometric
Stoichiometric Stoichiometric table for flow system
Stoichiometric table for flow system