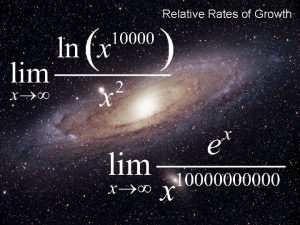Chemical Kinetics Expression of rates Stoichiometric relationships of
![Instantaneous Rate: Rate of decrease in [Phenolphthalein] Instantaneous Rate: Rate of decrease in [Phenolphthalein]](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-5.jpg)
![Rate Law Rate = k[NO 2]n • The concentrations of the products do not Rate Law Rate = k[NO 2]n • The concentrations of the products do not](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-13.jpg)
![Rate Law Rate = k[NO 2]n • The value of the exponent n must Rate Law Rate = k[NO 2]n • The value of the exponent n must](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-14.jpg)

![Graphical Method for Zero-Order Reaction D[R] = -k. Dt, and [R]t = [R]0 = Graphical Method for Zero-Order Reaction D[R] = -k. Dt, and [R]t = [R]0 =](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-34.jpg)
![Graph of Zero-order Reactions • Plot of [R]t versus t: slope = -k [R]t Graph of Zero-order Reactions • Plot of [R]t versus t: slope = -k [R]t](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-36.jpg)
![Graph of First Order Reactions Plot of ln]R]t versus t: slope = -k ln[R]t Graph of First Order Reactions Plot of ln]R]t versus t: slope = -k ln[R]t](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-38.jpg)
![Plots of [A] and ln[A] versus time for First Order Reactions Plots of [A] and ln[A] versus time for First Order Reactions](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-39.jpg)

![Graph of Second-order Reactions • Plot of 1/[R]t versus time: slope = k time Graph of Second-order Reactions • Plot of 1/[R]t versus time: slope = k time](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-43.jpg)
![Plots of ln[Concentration] versus time Plots of ln[Concentration] versus time](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-45.jpg)
![Plots of 1/[Concn. ] versus time Plots of 1/[Concn. ] versus time](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-46.jpg)
![Half-Lives of Reactions • For zero-order reaction: t 1/2 = [R]0/2 k; • For Half-Lives of Reactions • For zero-order reaction: t 1/2 = [R]0/2 k; • For](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-48.jpg)


![Exercise Consider the reaction a. A Products. [A]0 = 5. 0 M and k Exercise Consider the reaction a. A Products. [A]0 = 5. 0 M and k](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-52.jpg)



- Slides: 90

Chemical Kinetics • Expression of rates. • Stoichiometric relationships of rates of different substances in a reaction. • Determination of reaction orders, rate laws, and rate constant by method of initial rate. • Determination of rate laws by graphical or integration method. • Determination of half-lives • Determination of activation energy • Elementary steps and reaction mechanism • Effect of catalysts

Chemical Kinetics • The study of reaction rates; – How fast does a reaction proceeds and what factors affecting it; – A measure of the change of the concentration of a reactant (or a product) as a function of time. • The study of rate yields information on the mechanism by which a reaction occurs at molecular level.

Types of Rates • Initial Rates – Rates measured at the beginning of the reaction, which is dependent on the initial concentrations of reactants. • Instantaneous Rates – Rates measured at any point during the reaction. • Average Rates – An overall rate measured over a period or time interval.

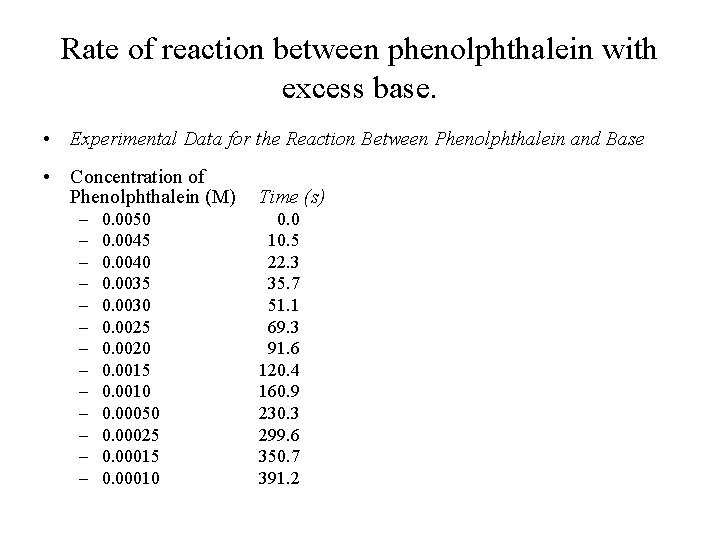
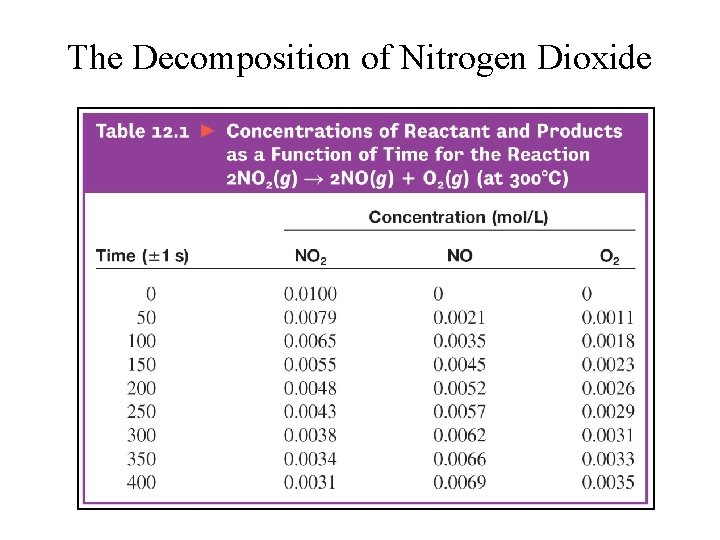
Rate of reaction between phenolphthalein with excess base. • Experimental Data for the Reaction Between Phenolphthalein and Base • Concentration of Phenolphthalein (M) – – – – 0. 0050 0. 0045 0. 0040 0. 0035 0. 0030 0. 0025 0. 0020 0. 0015 0. 0010 0. 00050 0. 00025 0. 00010 Time (s) 0. 0 10. 5 22. 3 35. 7 51. 1 69. 3 91. 6 120. 4 160. 9 230. 3 299. 6 350. 7 391. 2
![Instantaneous Rate Rate of decrease in Phenolphthalein Instantaneous Rate: Rate of decrease in [Phenolphthalein]](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-5.jpg)
Instantaneous Rate: Rate of decrease in [Phenolphthalein]

Instantaneous Rate • Value of the rate at a particular time. • Can be obtained by computing the slope of a line tangent to the curve at that point.

The Decomposition of Nitrogen Dioxide

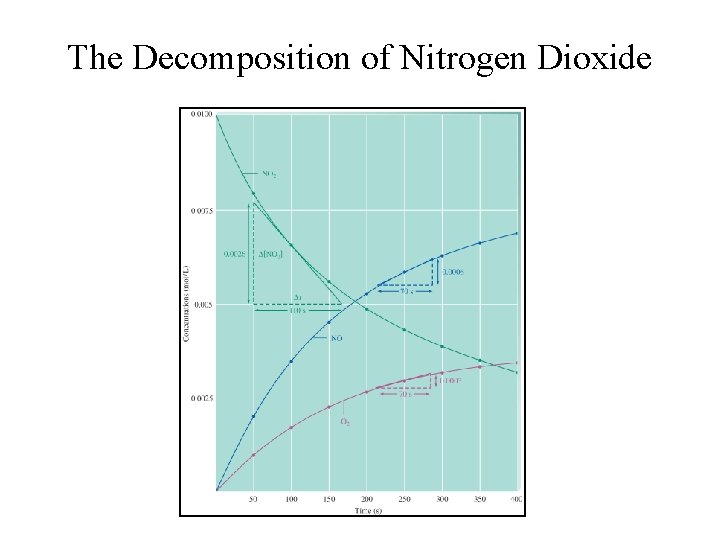
The Decomposition of Nitrogen Dioxide

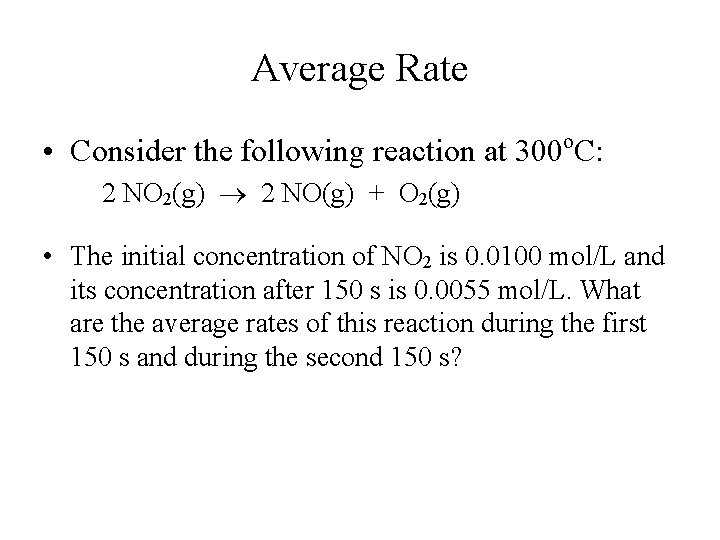
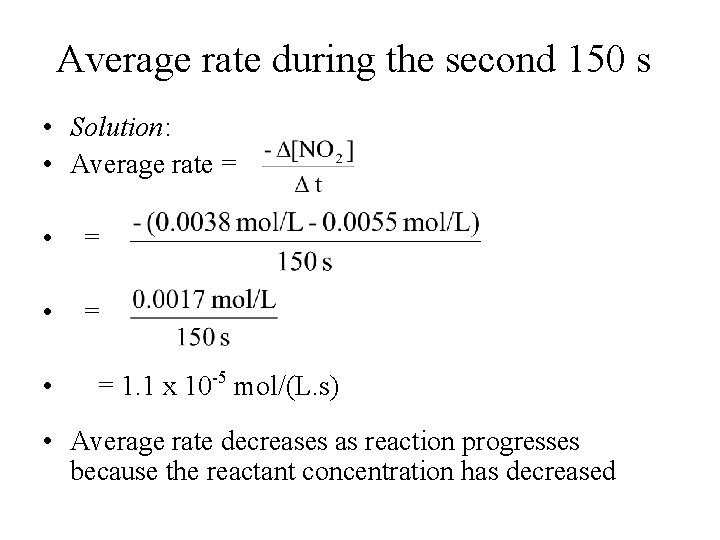
Average Rate o • Consider the following reaction at 300 C: 2 NO 2(g) 2 NO(g) + O 2(g) • The initial concentration of NO 2 is 0. 0100 mol/L and its concentration after 150 s is 0. 0055 mol/L. What are the average rates of this reaction during the first 150 s and during the second 150 s?

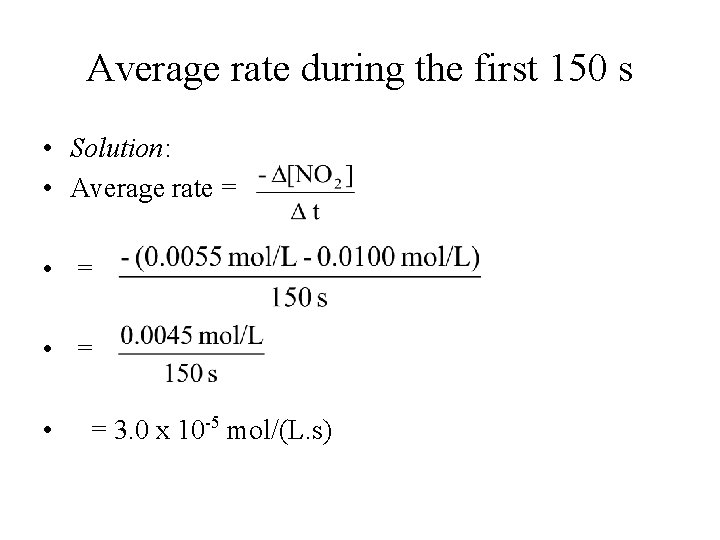
Average rate during the first 150 s • Solution: • Average rate = • = • = 3. 0 x 10 -5 mol/(L. s)

Average rate during the second 150 s • Solution: • Average rate = • = 1. 1 x 10 -5 mol/(L. s) • Average rate decreases as reaction progresses because the reactant concentration has decreased

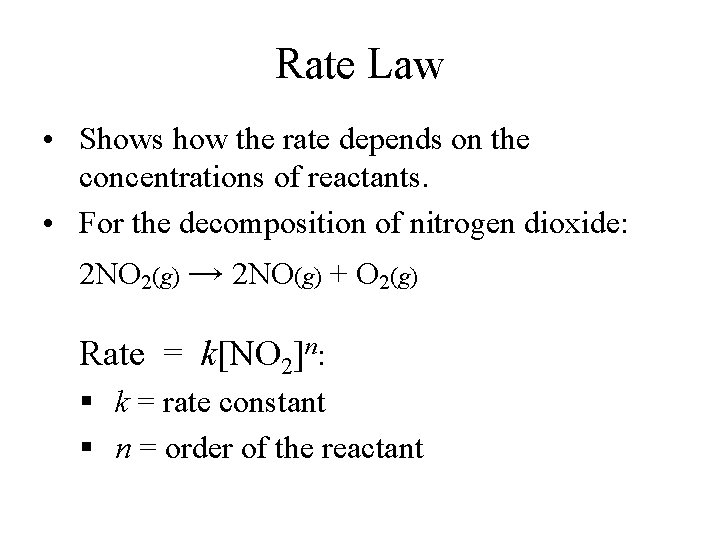
Rate Law • Shows how the rate depends on the concentrations of reactants. • For the decomposition of nitrogen dioxide: 2 NO 2(g) → 2 NO(g) + O 2(g) Rate = k[NO 2]n: § k = rate constant § n = order of the reactant
![Rate Law Rate kNO 2n The concentrations of the products do not Rate Law Rate = k[NO 2]n • The concentrations of the products do not](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-13.jpg)
Rate Law Rate = k[NO 2]n • The concentrations of the products do not appear in the rate law because the reaction rate is being studied under conditions where the reverse reaction does not contribute to the overall rate.
![Rate Law Rate kNO 2n The value of the exponent n must Rate Law Rate = k[NO 2]n • The value of the exponent n must](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-14.jpg)
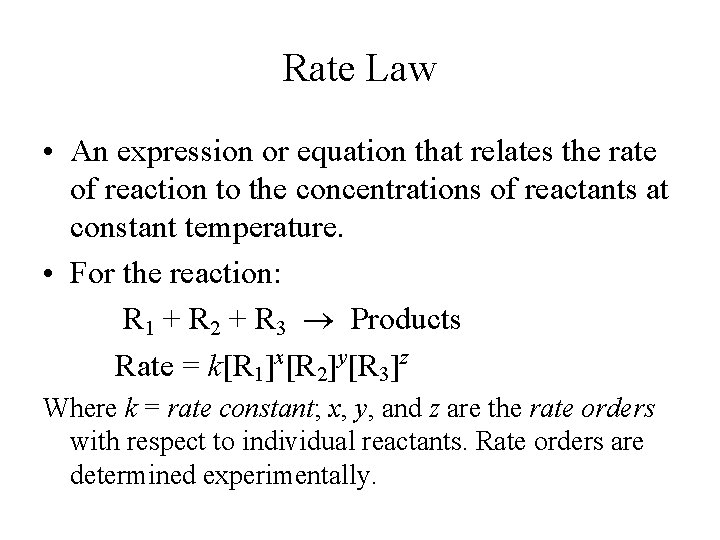
Rate Law Rate = k[NO 2]n • The value of the exponent n must be determined by experiment; it cannot be written from the balanced equation.

Rate Law • An expression or equation that relates the rate of reaction to the concentrations of reactants at constant temperature. • For the reaction: R 1 + R 2 + R 3 Products Rate = k[R 1]x[R 2]y[R 3]z Where k = rate constant; x, y, and z are the rate orders with respect to individual reactants. Rate orders are determined experimentally.

Types of Rate Laws • Differential Rate Law (rate law) – shows how the rate of a reaction depends on concentrations. • Integrated Rate Law – shows how the concentrations of species in the reaction depend on time.

Rate Laws: A Summary • Our rate laws involve only concentrations of reactants, because we typically consider reactions only under conditions where the reverse reaction is unimportant,

Rate Laws: A Summary • Experimental convenience usually dictates which type of rate law is determined experimentally. • Knowing the rate law for a reaction is important mainly because we can usually infer the individual steps involved in the reaction from the specific form of the rate law.

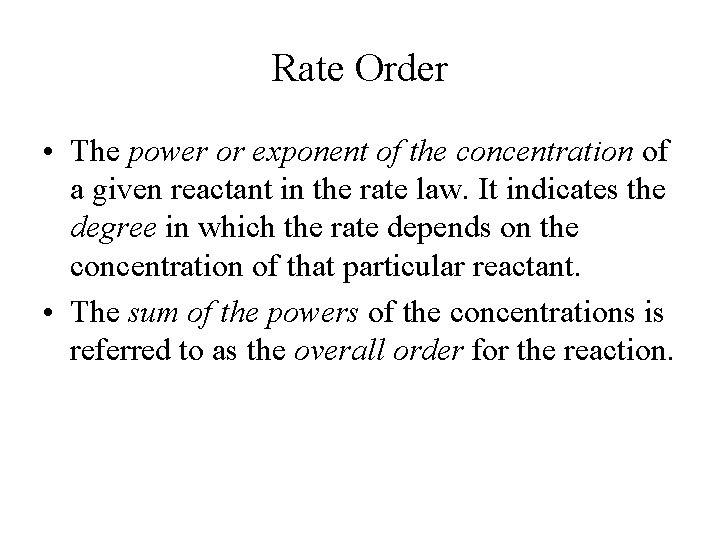
Rate Order • The power or exponent of the concentration of a given reactant in the rate law. It indicates the degree in which the rate depends on the concentration of that particular reactant. • The sum of the powers of the concentrations is referred to as the overall order for the reaction.

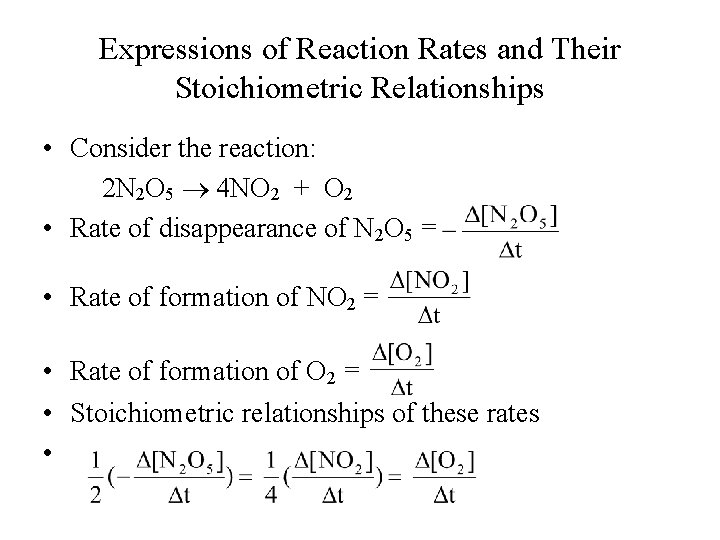
Expressions of Reaction Rates and Their Stoichiometric Relationships • Consider the reaction: 2 N 2 O 5 4 NO 2 + O 2 • Rate of disappearance of N 2 O 5 = • Rate of formation of NO 2 = • Rate of formation of O 2 = • Stoichiometric relationships of these rates •

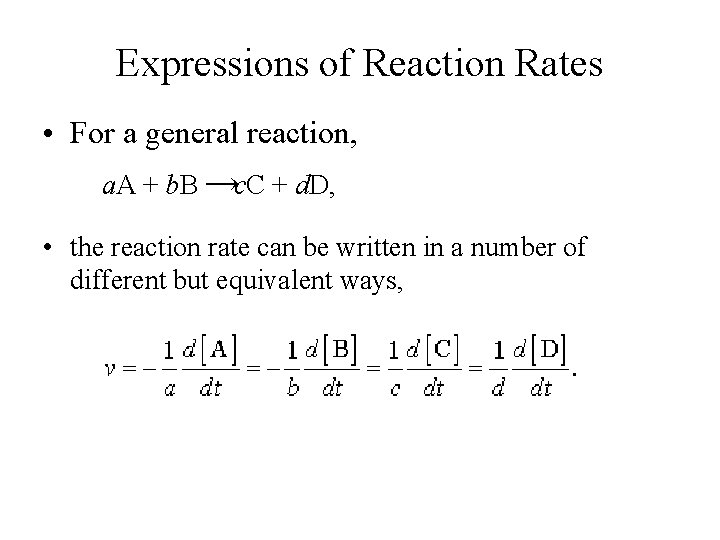
Expressions of Reaction Rates • For a general reaction, a. A + b. B →c. C + d. D, • the reaction rate can be written in a number of different but equivalent ways,

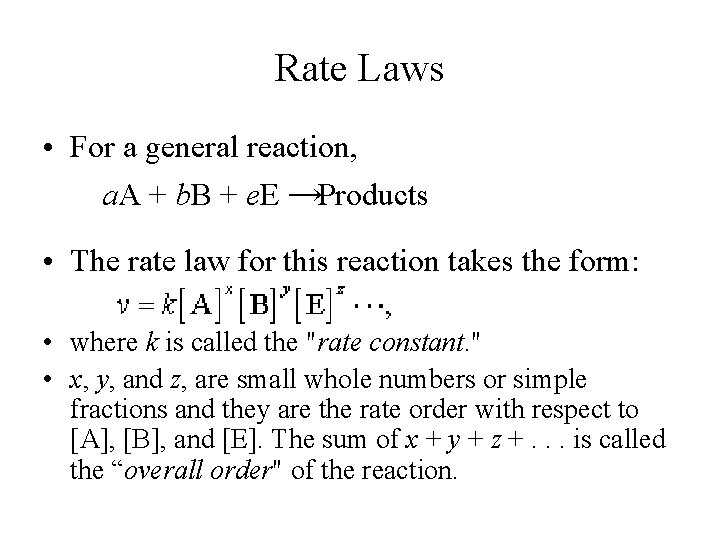
Rate Laws • For a general reaction, a. A + b. B + e. E →Products • The rate law for this reaction takes the form: • where k is called the "rate constant. " • x, y, and z, are small whole numbers or simple fractions and they are the rate order with respect to [A], [B], and [E]. The sum of x + y + z +. . . is called the “overall order" of the reaction.

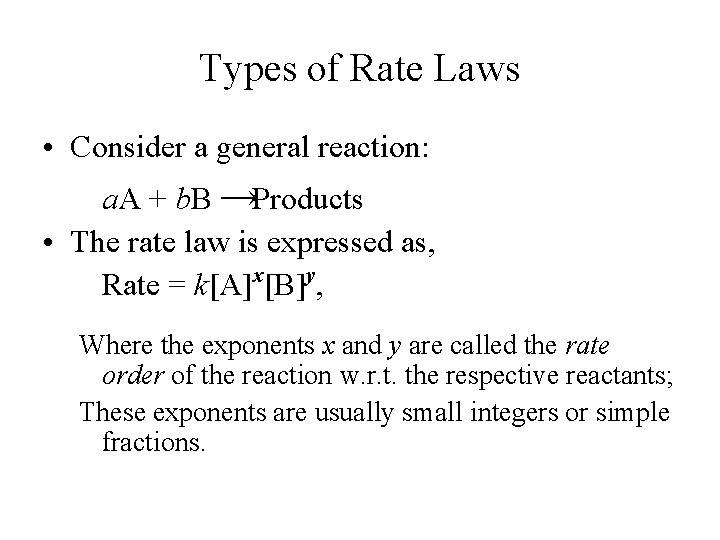
Types of Rate Laws • Consider a general reaction: a. A + b. B →Products • The rate law is expressed as, Rate = k[A]x[B]y, Where the exponents x and y are called the rate order of the reaction w. r. t. the respective reactants; These exponents are usually small integers or simple fractions.

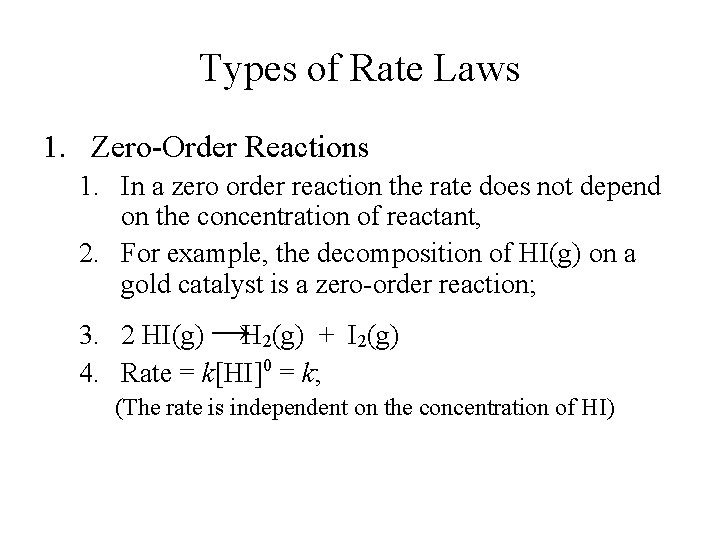
Types of Rate Laws 1. Zero-Order Reactions 1. In a zero order reaction the rate does not depend on the concentration of reactant, 2. For example, the decomposition of HI(g) on a gold catalyst is a zero-order reaction; 3. 2 HI(g) →H 2(g) + I 2(g) 4. Rate = k[HI]0 = k; (The rate is independent on the concentration of HI)

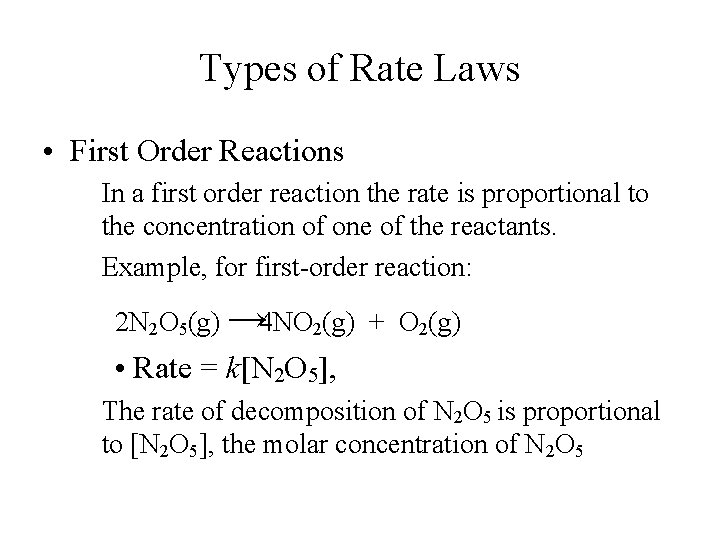
Types of Rate Laws • First Order Reactions In a first order reaction the rate is proportional to the concentration of one of the reactants. Example, for first-order reaction: 2 N 2 O 5(g) → 4 NO 2(g) + O 2(g) • Rate = k[N 2 O 5], The rate of decomposition of N 2 O 5 is proportional to [N 2 O 5], the molar concentration of N 2 O 5

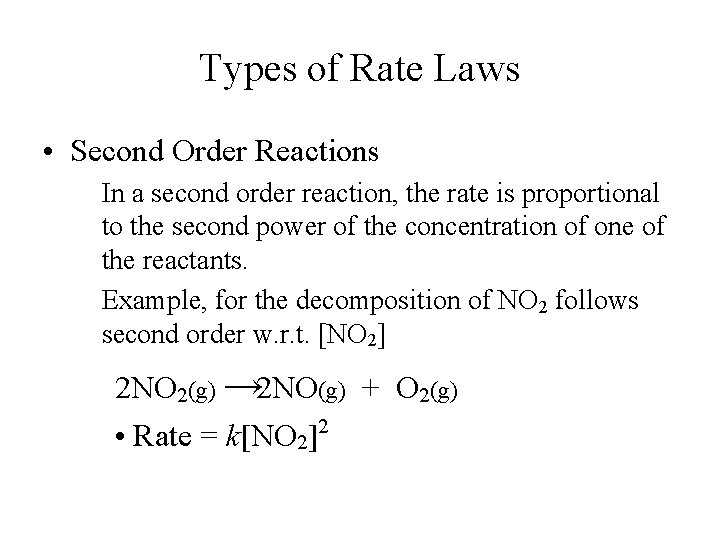
Types of Rate Laws • Second Order Reactions In a second order reaction, the rate is proportional to the second power of the concentration of one of the reactants. Example, for the decomposition of NO 2 follows second order w. r. t. [NO 2] 2 NO 2(g) → 2 NO(g) + O 2(g) • Rate = k[NO 2]2

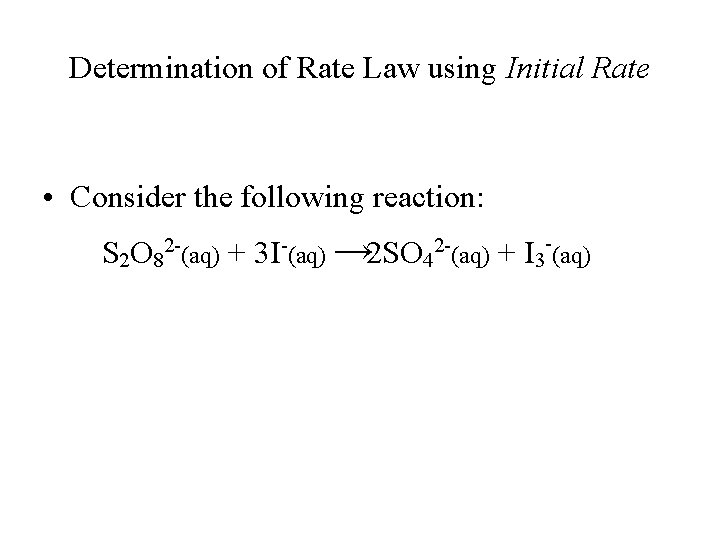
Determination of Rate Law using Initial Rate • Consider the following reaction: S 2 O 82 -(aq) + 3 I-(aq) → 2 SO 42 -(aq) + I 3 -(aq)

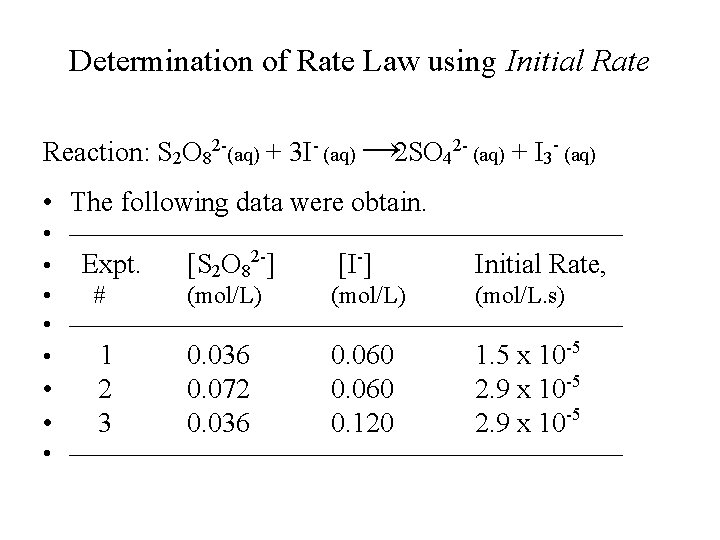
Determination of Rate Law using Initial Rate Reaction: S 2 O 82 -(aq) + 3 I- (aq) → 2 SO 42 - (aq) + I 3 - (aq) • The following data were obtain. • • Expt. [S 2 O 82 -] [I-] Initial Rate, • # (mol/L) (mol/L. s) • • 1 0. 036 0. 060 1. 5 x 10 -5 • • 2 3 0. 072 0. 036 0. 060 0. 120 2. 9 x 10 -5 •

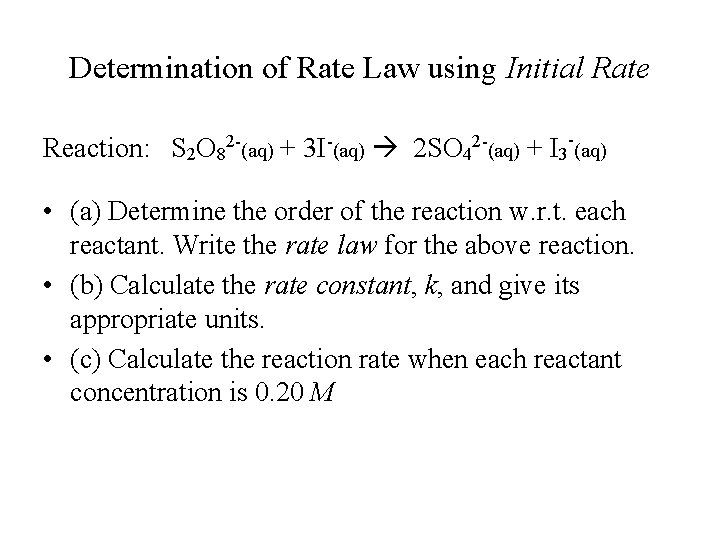
Determination of Rate Law using Initial Rate Reaction: S 2 O 82 -(aq) + 3 I-(aq) 2 SO 42 -(aq) + I 3 -(aq) • (a) Determine the order of the reaction w. r. t. each reactant. Write the rate law for the above reaction. • (b) Calculate the rate constant, k, and give its appropriate units. • (c) Calculate the reaction rate when each reactant concentration is 0. 20 M

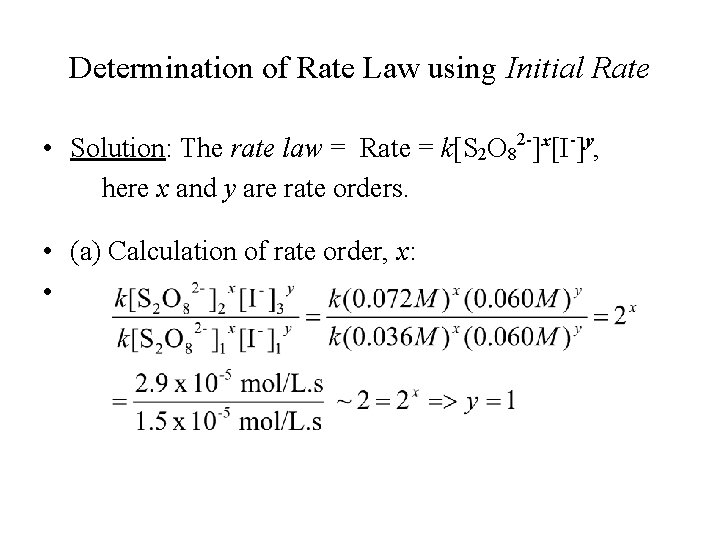
Determination of Rate Law using Initial Rate • Solution: The rate law = Rate = k[S 2 O 82 -]x[I-]y, here x and y are rate orders. • (a) Calculation of rate order, x: •

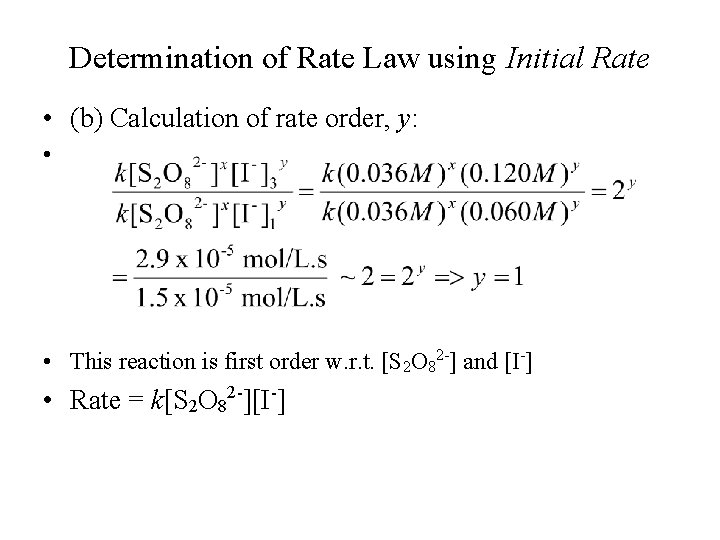
Determination of Rate Law using Initial Rate • (b) Calculation of rate order, y: • • This reaction is first order w. r. t. [S 2 O 82 -] and [I-] • Rate = k[S 2 O 82 -][I-]

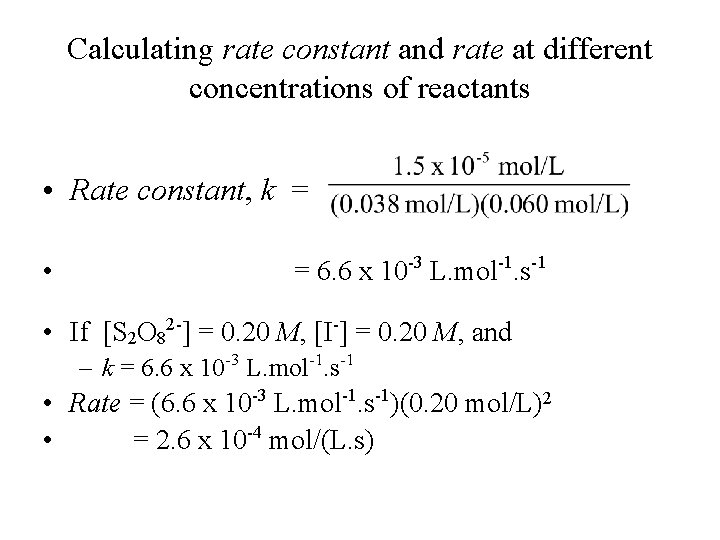
Calculating rate constant and rate at different concentrations of reactants • Rate constant, k = • = 6. 6 x 10 -3 L. mol-1. s-1 • If [S 2 O 82 -] = 0. 20 M, [I-] = 0. 20 M, and – k = 6. 6 x 10 -3 L. mol-1. s-1 • Rate = (6. 6 x 10 -3 L. mol-1. s-1)(0. 20 mol/L)2 • = 2. 6 x 10 -4 mol/(L. s)

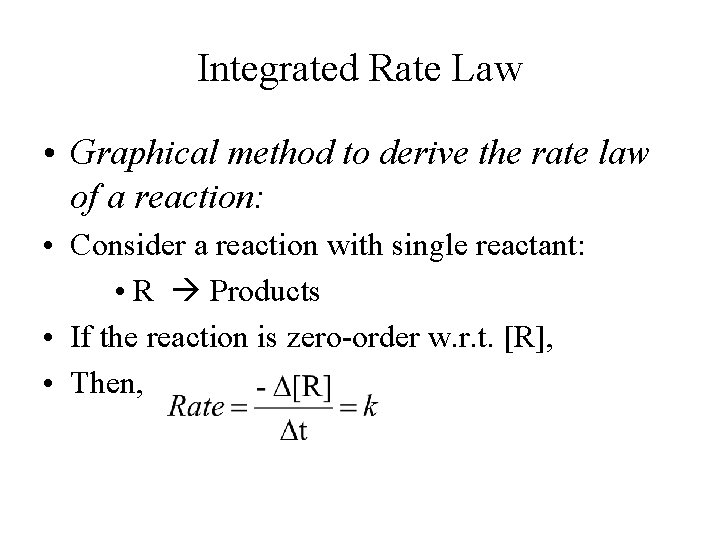
Integrated Rate Law • Graphical method to derive the rate law of a reaction: • Consider a reaction with single reactant: • R Products • If the reaction is zero-order w. r. t. [R], • Then,
![Graphical Method for ZeroOrder Reaction DR k Dt and Rt R0 Graphical Method for Zero-Order Reaction D[R] = -k. Dt, and [R]t = [R]0 =](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-34.jpg)
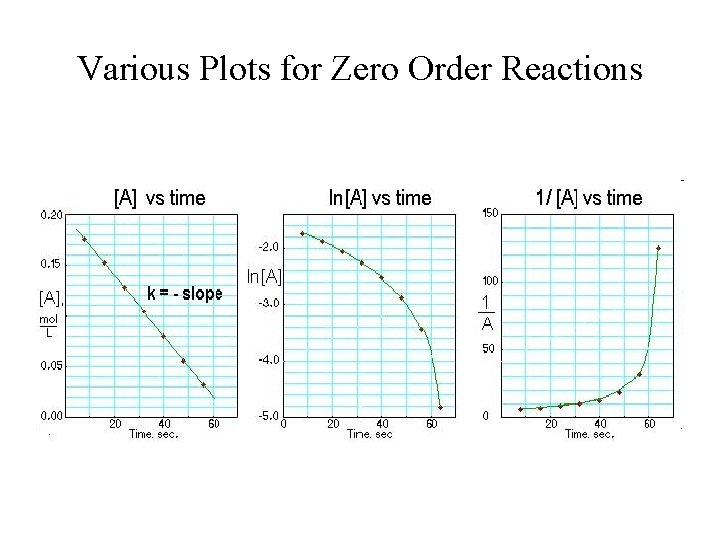
Graphical Method for Zero-Order Reaction D[R] = -k. Dt, and [R]t = [R]0 = kt; A plot of [R]t versus t yields a straight line with k = -slope.

Various Plots for Zero Order Reactions
![Graph of Zeroorder Reactions Plot of Rt versus t slope k Rt Graph of Zero-order Reactions • Plot of [R]t versus t: slope = -k [R]t](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-36.jpg)
Graph of Zero-order Reactions • Plot of [R]t versus t: slope = -k [R]t t

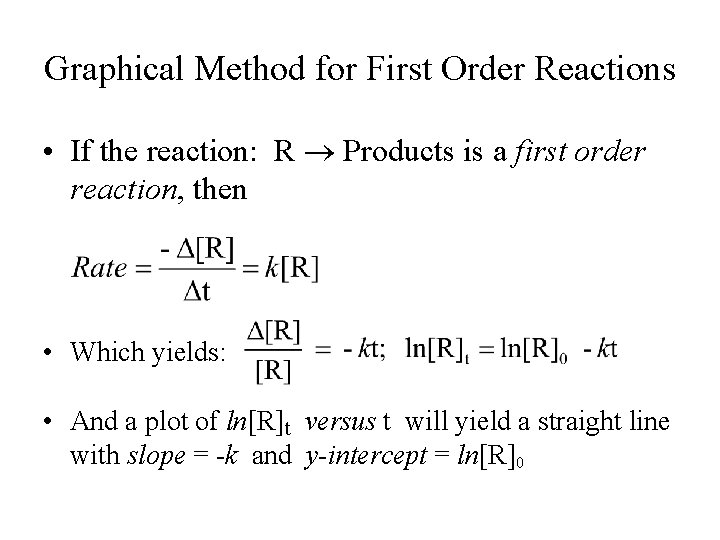
Graphical Method for First Order Reactions • If the reaction: R Products is a first order reaction, then • Which yields: • And a plot of ln[R]t versus t will yield a straight line with slope = -k and y-intercept = ln[R]0
![Graph of First Order Reactions Plot of lnRt versus t slope k lnRt Graph of First Order Reactions Plot of ln]R]t versus t: slope = -k ln[R]t](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-38.jpg)
Graph of First Order Reactions Plot of ln]R]t versus t: slope = -k ln[R]t t
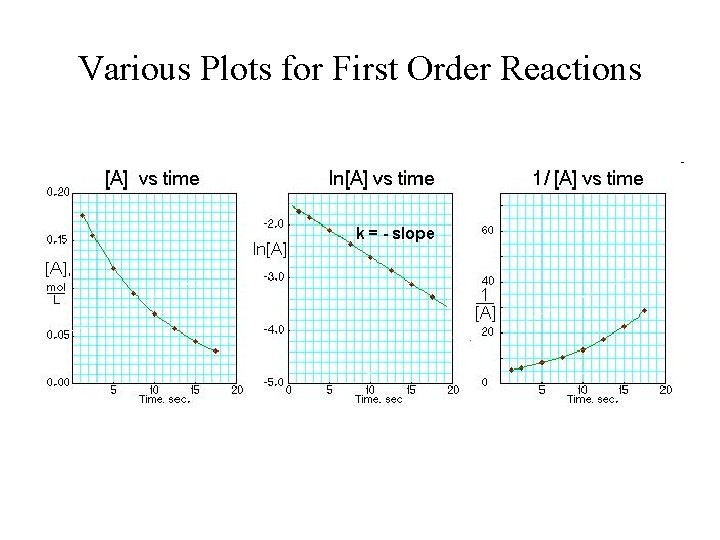
![Plots of A and lnA versus time for First Order Reactions Plots of [A] and ln[A] versus time for First Order Reactions](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-39.jpg)
Plots of [A] and ln[A] versus time for First Order Reactions

Various Plots for First Order Reactions

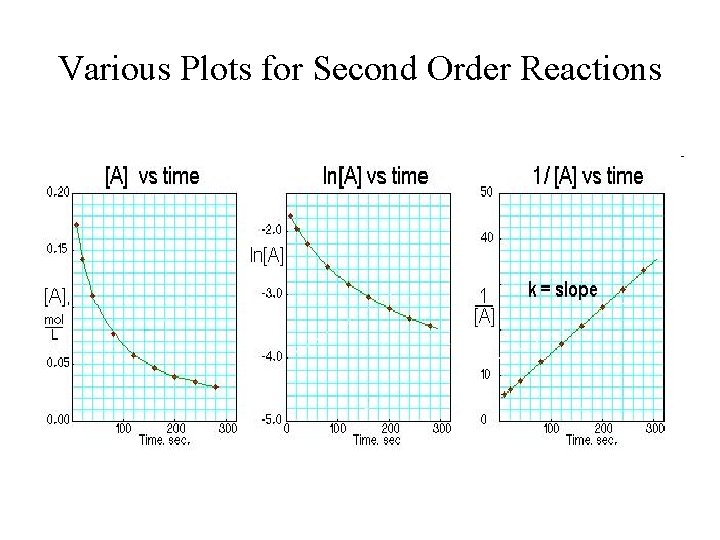
Graphical Method for Second Order Reactions • If the reaction: R Products follows secondorder kinetics, then • or • • and • A plot of 1/[R]t versus t will yield a straight line with slope = k and y-intercept = 1/[R]0

Various Plots for Second Order Reactions
![Graph of Secondorder Reactions Plot of 1Rt versus time slope k time Graph of Second-order Reactions • Plot of 1/[R]t versus time: slope = k time](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-43.jpg)
Graph of Second-order Reactions • Plot of 1/[R]t versus time: slope = k time

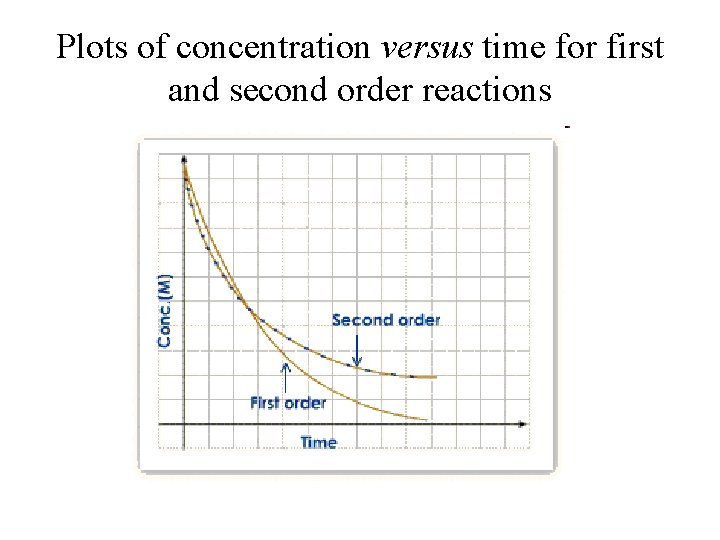
Plots of concentration versus time for first and second order reactions
![Plots of lnConcentration versus time Plots of ln[Concentration] versus time](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-45.jpg)
Plots of ln[Concentration] versus time
![Plots of 1Concn versus time Plots of 1/[Concn. ] versus time](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-46.jpg)
Plots of 1/[Concn. ] versus time

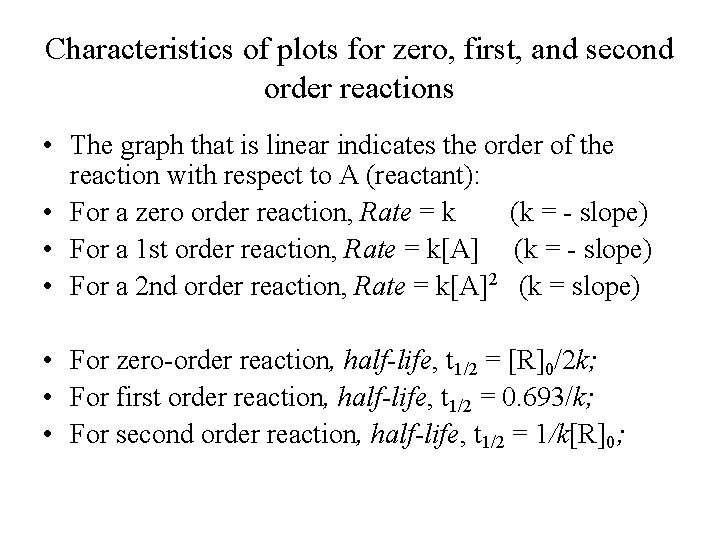
Characteristics of plots for zero, first, and second order reactions • The graph that is linear indicates the order of the reaction with respect to A (reactant): • For a zero order reaction, Rate = k (k = - slope) • For a 1 st order reaction, Rate = k[A] (k = - slope) • For a 2 nd order reaction, Rate = k[A]2 (k = slope) • For zero-order reaction, half-life, t 1/2 = [R]0/2 k; • For first order reaction, half-life, t 1/2 = 0. 693/k; • For second order reaction, half-life, t 1/2 = 1/k[R]0;
![HalfLives of Reactions For zeroorder reaction t 12 R02 k For Half-Lives of Reactions • For zero-order reaction: t 1/2 = [R]0/2 k; • For](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-48.jpg)
Half-Lives of Reactions • For zero-order reaction: t 1/2 = [R]0/2 k; • For first-order reaction: t 1/2 = 0. 693/k; • For second-order reaction: t 1/2 = 1/(k[R]0) • Note: For first-order reaction, the half-life is independent of the concentration of reactant, but for zero-order and second-order reactions, the half-lives are dependent on the initial concentrations of the reactants.

Half-Life of Reactions

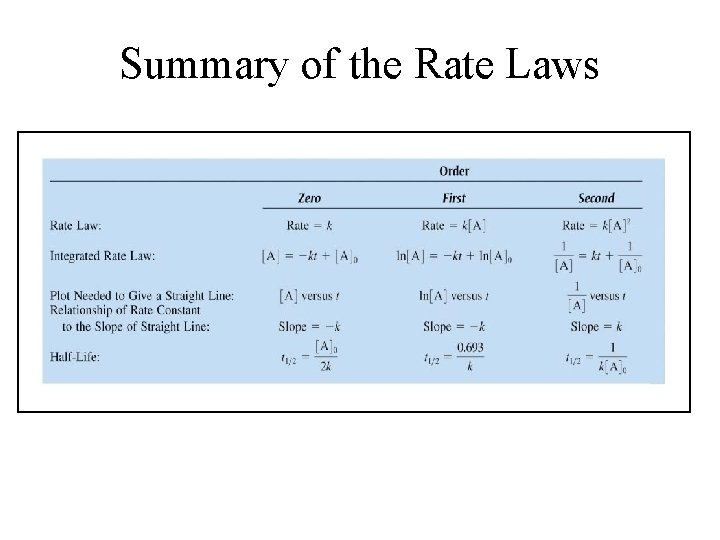
Rate Laws

Summary of the Rate Laws
![Exercise Consider the reaction a A Products A0 5 0 M and k Exercise Consider the reaction a. A Products. [A]0 = 5. 0 M and k](https://slidetodoc.com/presentation_image_h2/0c51c2801e9da21e843653756951fa93/image-52.jpg)
Exercise Consider the reaction a. A Products. [A]0 = 5. 0 M and k = 1. 0 x 10– 2 (assume the units are appropriate for each case). Calculate [A] after 30. 0 seconds have passed, assuming the reaction is: a) Zero order b) First order c) Second order 4. 7 M 3. 7 M 2. 0 M

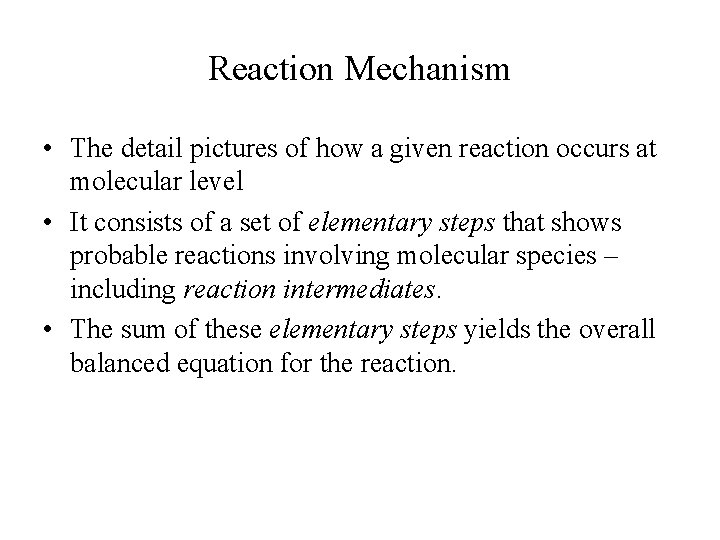
Reaction Mechanism • The detail pictures of how a given reaction occurs at molecular level • It consists of a set of elementary steps that shows probable reactions involving molecular species – including reaction intermediates. • The sum of these elementary steps yields the overall balanced equation for the reaction.

Elementary Steps • For example, the overall reaction: 2 A + B C + D • may involves the following elementary steps in its mechanism: • Step-1: A + B X; • Step-2: X + A Y; • Step-3: Y C+ D • Overall reaction: 2 A + B C + D;

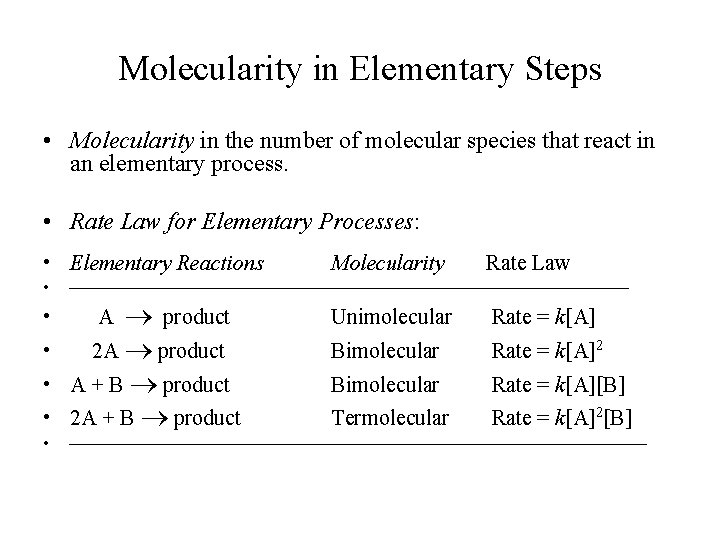
Molecularity in Elementary Steps • Molecularity in the number of molecular species that react in an elementary process. • Rate Law for Elementary Processes: • Elementary Reactions • Rate Law Unimolecular Rate = k[A] Bimolecular Rate = k[A]2 Bimolecular Rate = k[A][B] Termolecular Rate = k[A]2[B] • A product • 2 A product • A + B product • 2 A + B product • Molecularity

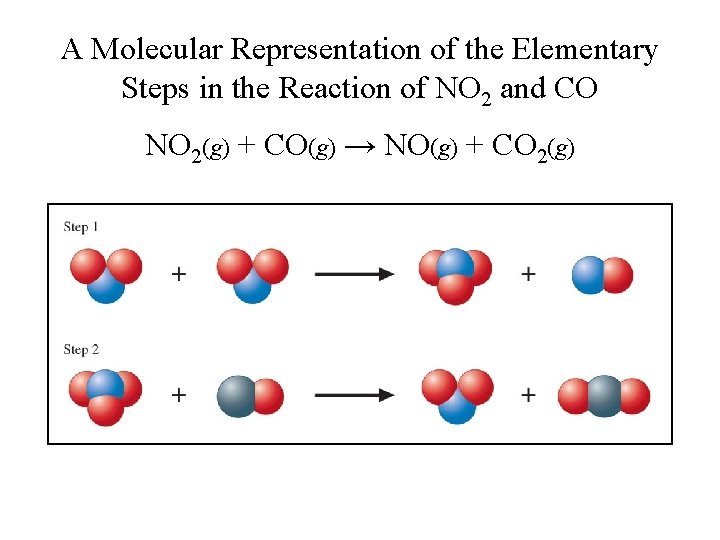
A Molecular Representation of the Elementary Steps in the Reaction of NO 2 and CO NO 2(g) + CO(g) → NO(g) + CO 2(g)

Reaction Mechanism • Step-1: NO 2 + NO 2 NO 3 + NO • Step-2: NO 3 + CO NO 2 + CO 2 • Overall: NO 2 + CO NO + CO 2 • The experimental rate law is Rate = k[NO 2]2 • Which implies that the above reaction is second-order w. r. t. NO 2 , but is zero-order in [CO].

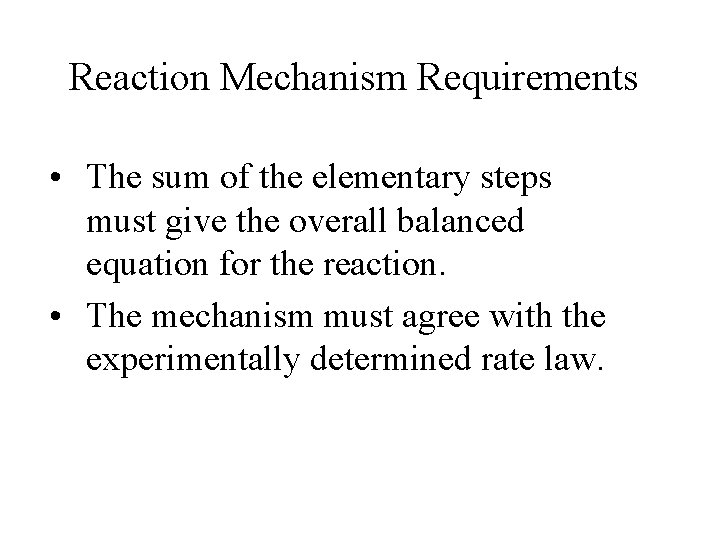
Reaction Mechanism Requirements • The sum of the elementary steps must give the overall balanced equation for the reaction. • The mechanism must agree with the experimentally determined rate law.

Decomposition of N 2 O 5

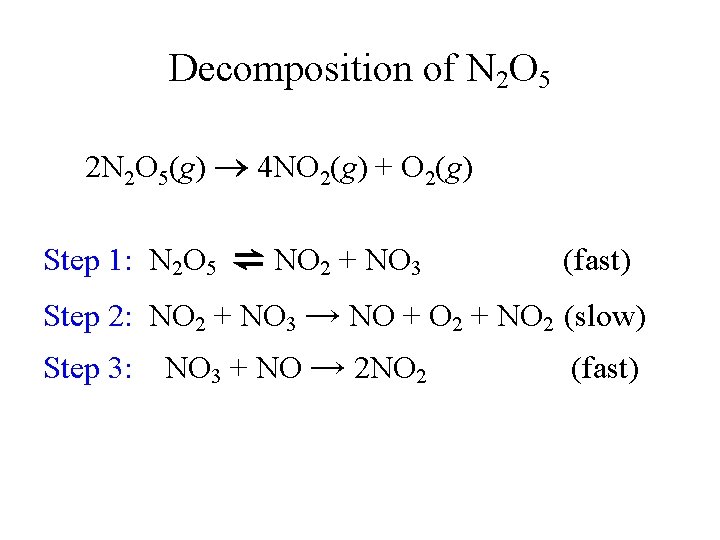
Decomposition of N 2 O 5 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) Step 1: N 2 O 5 ⇌ NO 2 + NO 3 (fast) Step 2: NO 2 + NO 3 → NO + O 2 + NO 2 (slow) Step 3: NO 3 + NO → 2 NO 2 (fast)

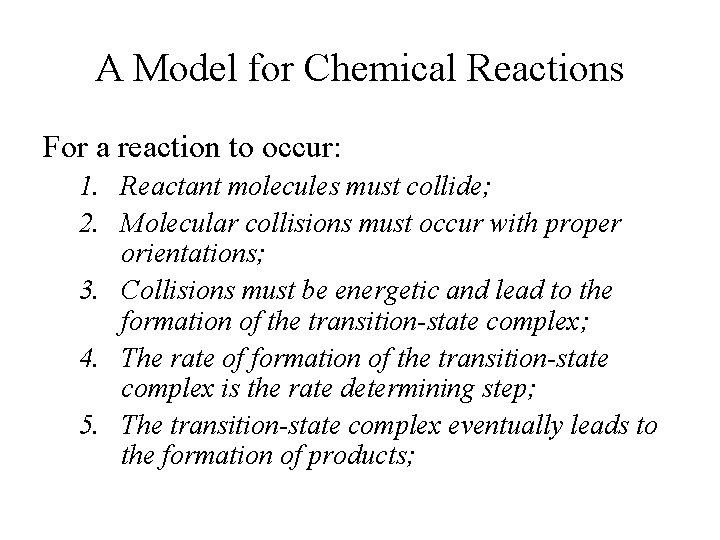
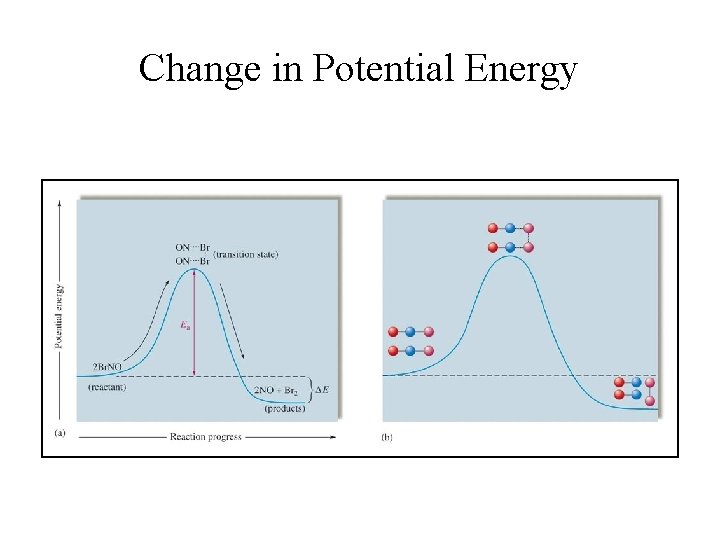
A Model for Chemical Reactions For a reaction to occur: 1. Reactant molecules must collide; 2. Molecular collisions must occur with proper orientations; 3. Collisions must be energetic and lead to the formation of the transition-state complex; 4. The rate of formation of the transition-state complex is the rate determining step; 5. The transition-state complex eventually leads to the formation of products;

A Model for Reaction Kinetics • All chemical reactions proceed through a transition-state complex; • An energy barrier called activation energy (Ea) must be overcome to change reactants to the transition-state. • The rate of formation of transition-state is the ratedetermining step for the overall reaction; • The rate of formation of transition-state is dependent: – on the frequency of effective molecular collisions, which depends on the reactants concentrations; – on the fraction of molecules with sufficient kinetic energy to overcome the energy barrier, and – on the reaction temperature

Transition States and Activation Energy

Change in Potential Energy

For Reactants to Form Products • Collision must involve enough energy to produce the reaction (must equal or exceed the activation energy). • Relative orientation of the reactants must allow formation of any new bonds necessary to produce products.

The Gas Phase Reaction of NO and Cl 2

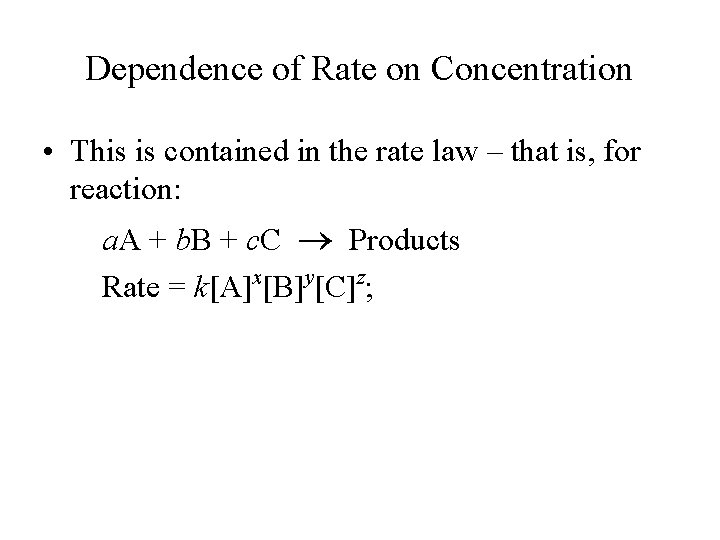
Dependence of Rate on Concentration • This is contained in the rate law – that is, for reaction: a. A + b. B + c. C Products Rate = k[A]x[B]y[C]z;

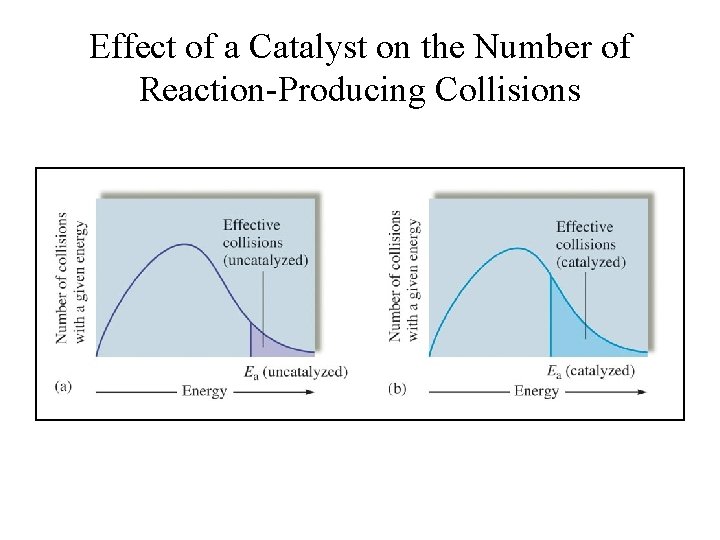
Dependence of Rate on Temperature • Rate depends on the fraction of “effective collisions” per unit time. – (Effective collisions are those with proper orientation and sufficient energy to overcome activation energy Ea barrier. • Thus rate depends on the activation energy and temperature, such that, – Higher activation energy implies high barrier and fewer reactant molecules will form the transition-state complex. This leads to a slower rate of reaction; – Higher temperature results in a larger fraction of reactant molecules with sufficient energy to overcome the energy barrier. This leads to a faster rate of reaction.

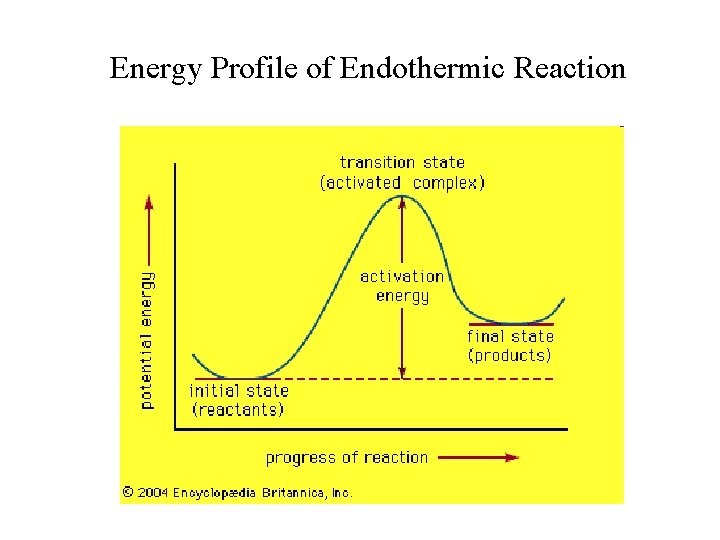
Energy Profile of Endothermic Reaction

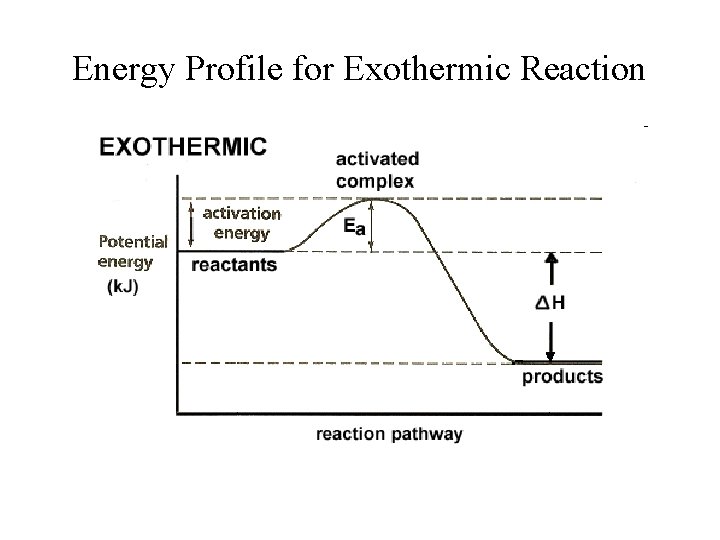
Energy Profile for Exothermic Reaction

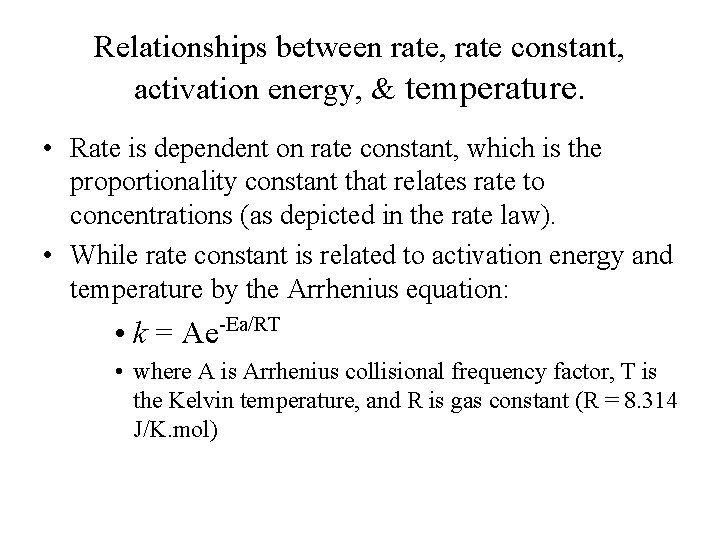
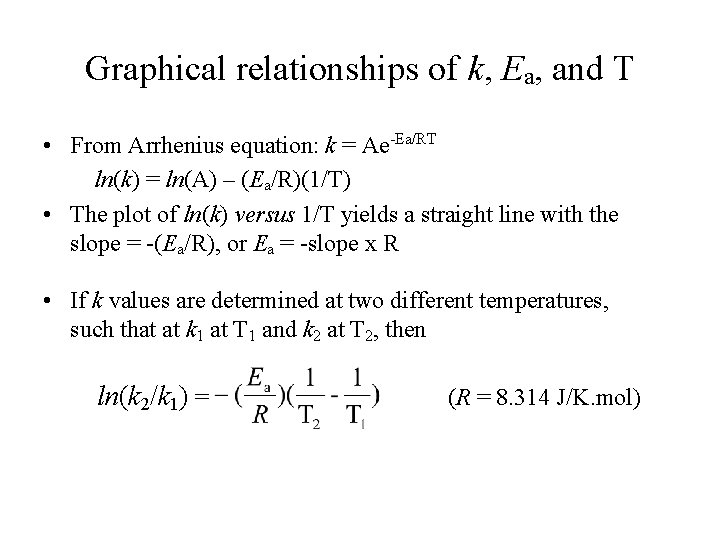
Relationships between rate, rate constant, activation energy, & temperature. • Rate is dependent on rate constant, which is the proportionality constant that relates rate to concentrations (as depicted in the rate law). • While rate constant is related to activation energy and temperature by the Arrhenius equation: • k = Ae-Ea/RT • where A is Arrhenius collisional frequency factor, T is the Kelvin temperature, and R is gas constant (R = 8. 314 J/K. mol)

Graphical relationships of k, Ea, and T • From Arrhenius equation: k = Ae-Ea/RT ln(k) = ln(A) – (Ea/R)(1/T) • The plot of ln(k) versus 1/T yields a straight line with the slope = -(Ea/R), or Ea = -slope x R • If k values are determined at two different temperatures, such that at k 1 at T 1 and k 2 at T 2, then ln(k 2/k 1) = (R = 8. 314 J/K. mol)

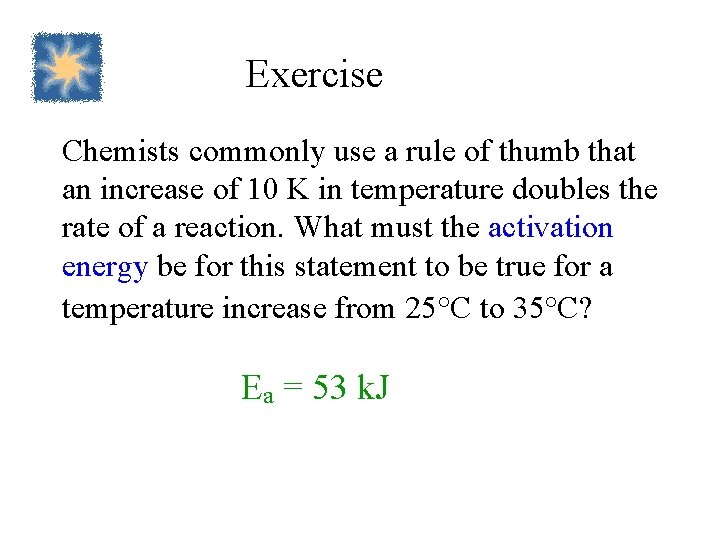
Exercise Chemists commonly use a rule of thumb that an increase of 10 K in temperature doubles the rate of a reaction. What must the activation energy be for this statement to be true for a temperature increase from 25°C to 35°C? Ea = 53 k. J

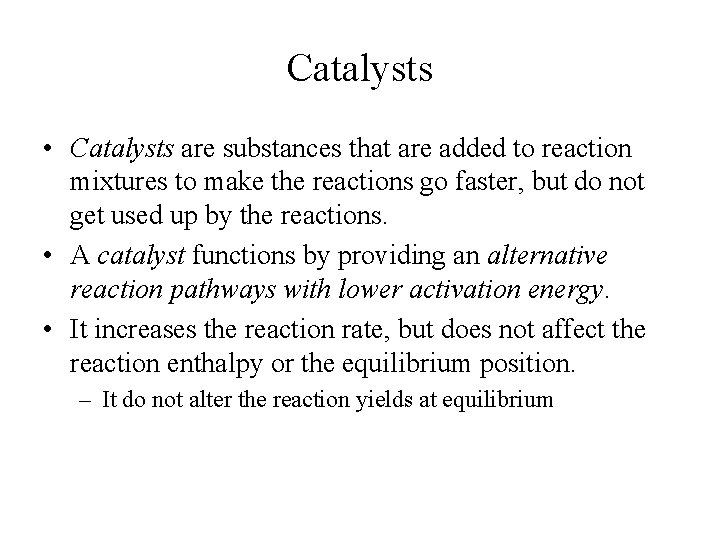
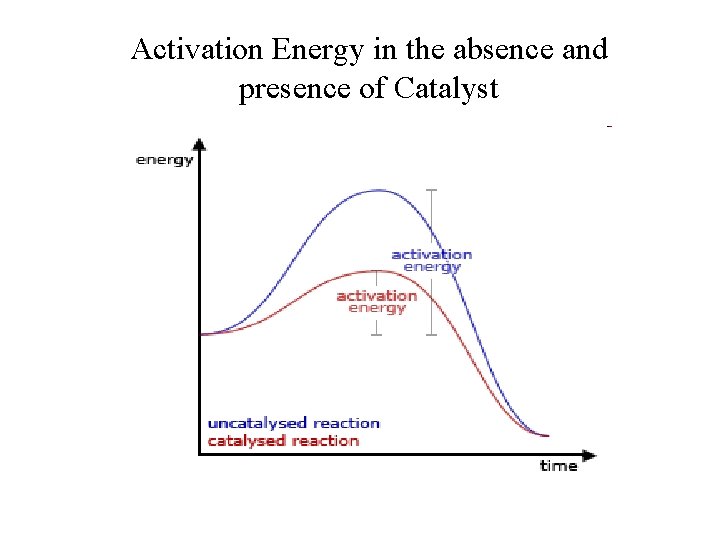
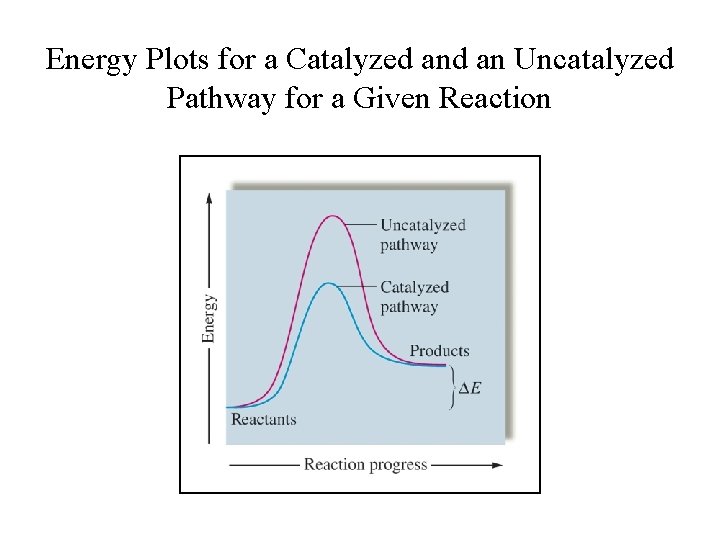
Catalysts • Catalysts are substances that are added to reaction mixtures to make the reactions go faster, but do not get used up by the reactions. • A catalyst functions by providing an alternative reaction pathways with lower activation energy. • It increases the reaction rate, but does not affect the reaction enthalpy or the equilibrium position. – It do not alter the reaction yields at equilibrium

Activation Energy in the absence and presence of Catalyst

Energy Plots for a Catalyzed an Uncatalyzed Pathway for a Given Reaction

Effect of a Catalyst on the Number of Reaction-Producing Collisions

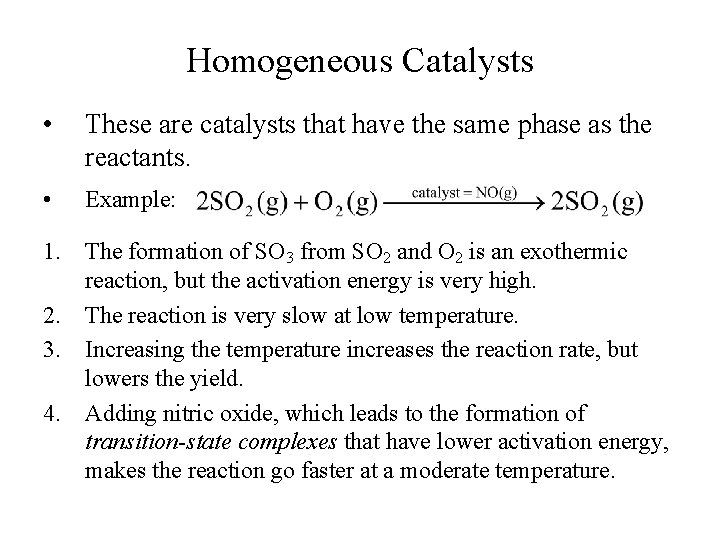
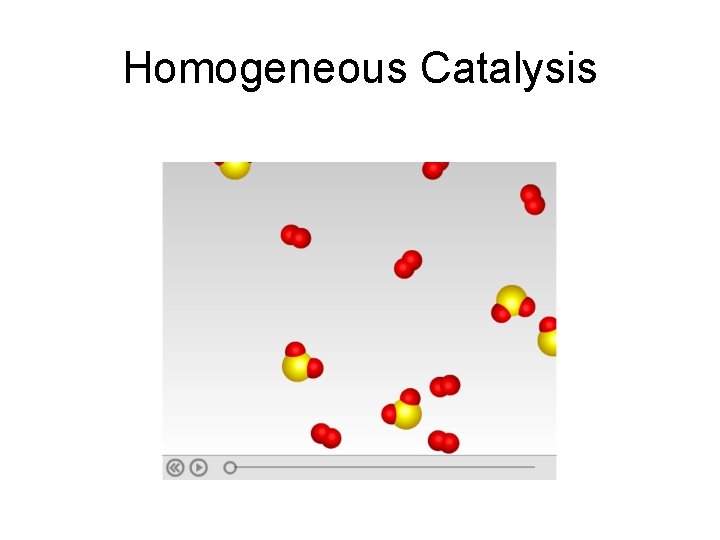
Homogeneous Catalysts • These are catalysts that have the same phase as the reactants. • Example: 1. The formation of SO 3 from SO 2 and O 2 is an exothermic reaction, but the activation energy is very high. 2. The reaction is very slow at low temperature. 3. Increasing the temperature increases the reaction rate, but lowers the yield. 4. Adding nitric oxide, which leads to the formation of transition-state complexes that have lower activation energy, makes the reaction go faster at a moderate temperature.

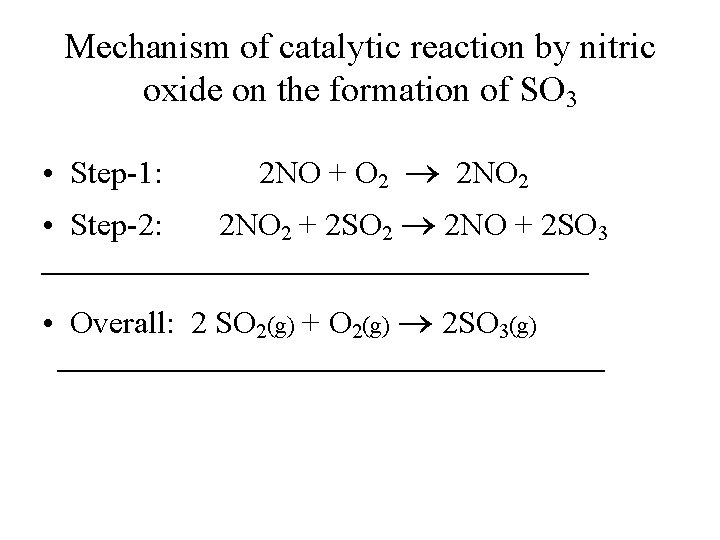
Mechanism of catalytic reaction by nitric oxide on the formation of SO 3 • Step-1: 2 NO + O 2 2 NO 2 • Step-2: 2 NO 2 + 2 SO 2 2 NO + 2 SO 3 • Overall: 2 SO 2(g) + O 2(g) 2 SO 3(g)

Homogeneous Catalysis

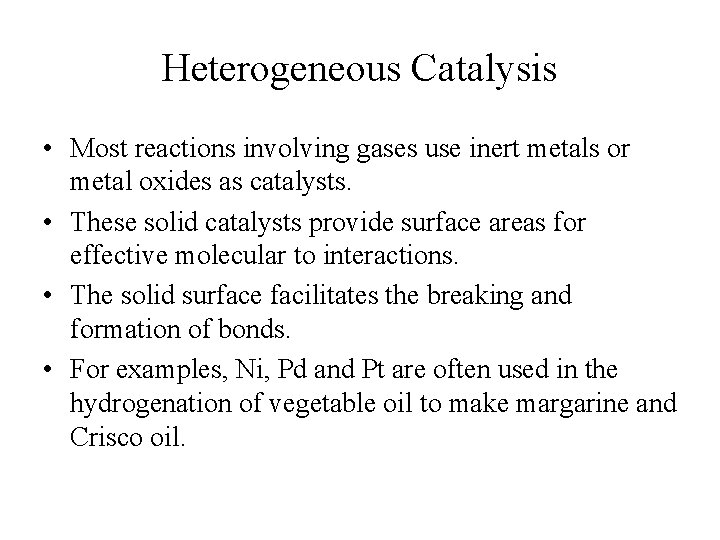
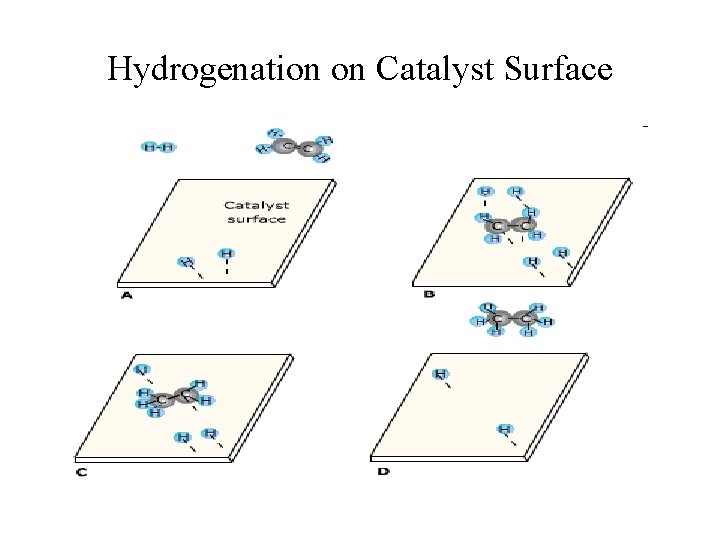
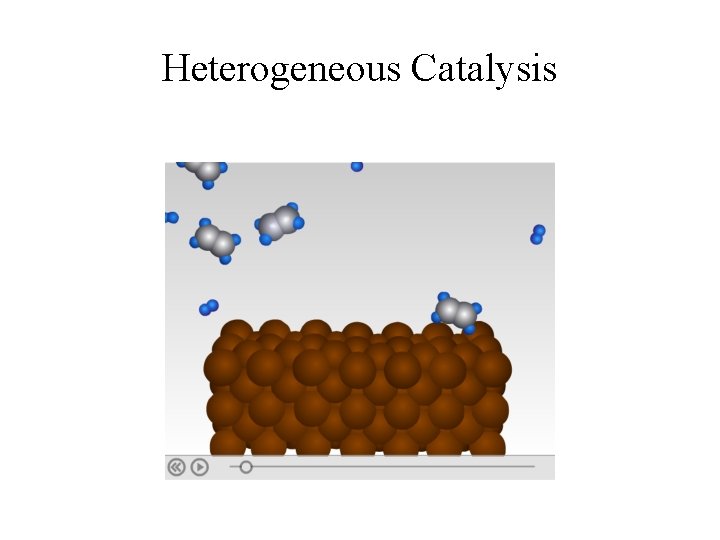
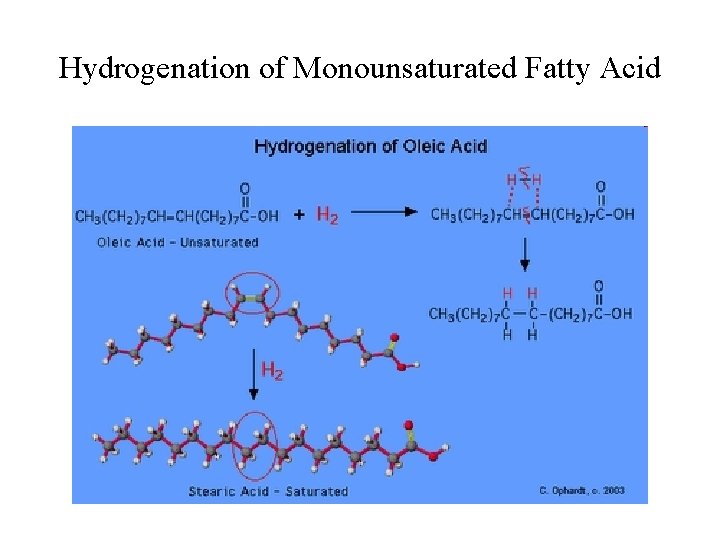
Heterogeneous Catalysis • Most reactions involving gases use inert metals or metal oxides as catalysts. • These solid catalysts provide surface areas for effective molecular to interactions. • The solid surface facilitates the breaking and formation of bonds. • For examples, Ni, Pd and Pt are often used in the hydrogenation of vegetable oil to make margarine and Crisco oil.

Hydrogenation on Catalyst Surface

Heterogeneous Catalysis

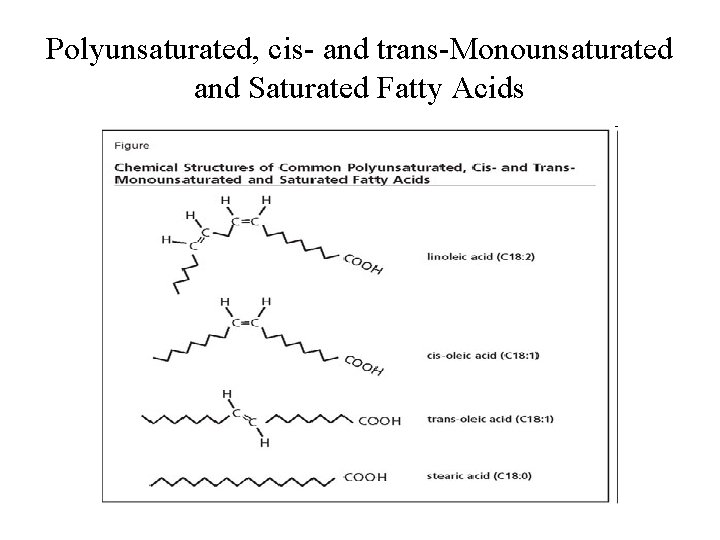
Polyunsaturated, cis- and trans-Monounsaturated and Saturated Fatty Acids

Hydrogenation of Monounsaturated Fatty Acid

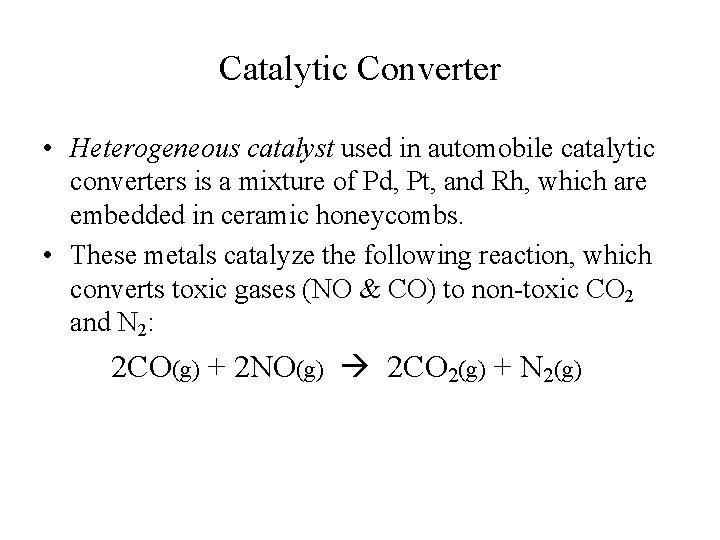
Catalytic Converter • Heterogeneous catalyst used in automobile catalytic converters is a mixture of Pd, Pt, and Rh, which are embedded in ceramic honeycombs. • These metals catalyze the following reaction, which converts toxic gases (NO & CO) to non-toxic CO 2 and N 2: 2 CO(g) + 2 NO(g) 2 CO 2(g) + N 2(g)

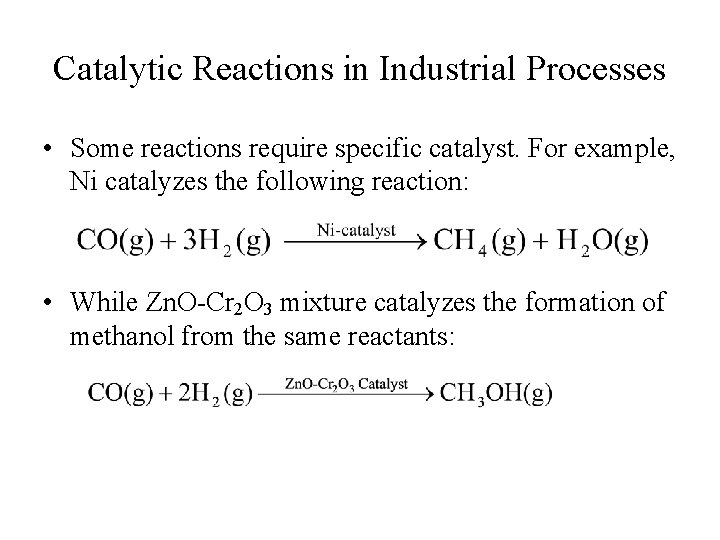
Catalytic Reactions in Industrial Processes • Some reactions require specific catalyst. For example, Ni catalyzes the following reaction: • While Zn. O-Cr 2 O 3 mixture catalyzes the formation of methanol from the same reactants:

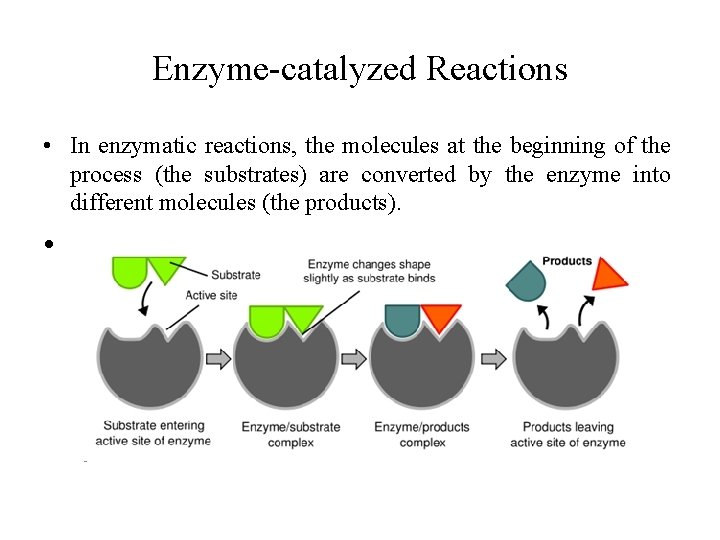
Enzyme-catalyzed Reactions • In enzymatic reactions, the molecules at the beginning of the process (the substrates) are converted by the enzyme into different molecules (the products). •

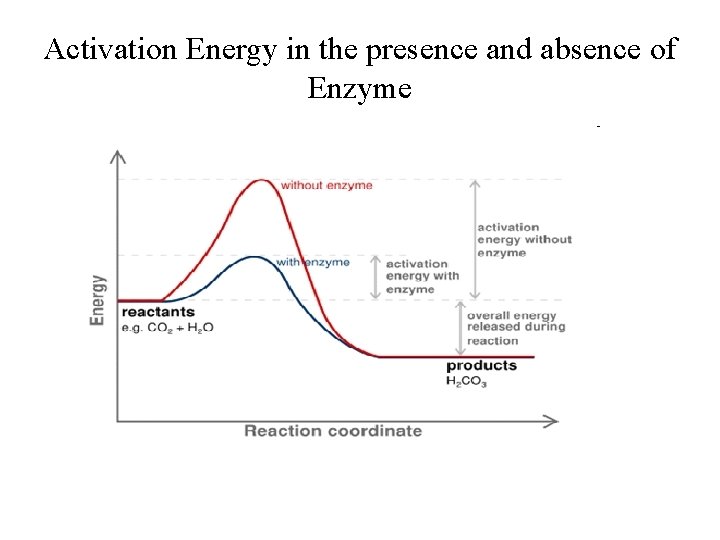
Activation Energy in the presence and absence of Enzyme

End of Slide
 Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Ratios and proportions guided notes
Ratios and proportions guided notes Half life chemical kinetics
Half life chemical kinetics Steady state approximation
Steady state approximation Chemical kinetics definition
Chemical kinetics definition Molecularity of reaction
Molecularity of reaction Chemical kinetics experiment
Chemical kinetics experiment Grade 11 chemistry unit 4
Grade 11 chemistry unit 4 Applications of chemical kinetics
Applications of chemical kinetics Quadratic equation examples
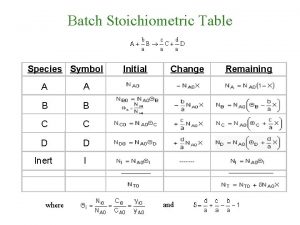
Quadratic equation examples Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric table
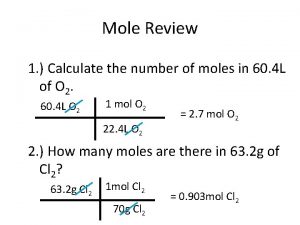
Stoichiometric table Calculate moles
Calculate moles Stoichiometric table for flow system
Stoichiometric table for flow system All stoichiometric calculations begin with a
All stoichiometric calculations begin with a Stoichiometric gasoline
Stoichiometric gasoline Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometry and stoichiometric calculations
Stoichiometry and stoichiometric calculations Mass to mass equation
Mass to mass equation Stoichiometric coefficient
Stoichiometric coefficient Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric table for reversible reaction
Stoichiometric table for reversible reaction Stoichiometric point definition
Stoichiometric point definition What is stoichiometric
What is stoichiometric Stoichiometry
Stoichiometry Combustion example
Combustion example Quantitative relationships in chemical equations
Quantitative relationships in chemical equations Mass relationships in chemical reactions
Mass relationships in chemical reactions Mole relationships in chemical equations
Mole relationships in chemical equations Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Fermenter
Fermenter Kinematics of rigid bodies problems and solutions
Kinematics of rigid bodies problems and solutions Ap chemistry kinetics
Ap chemistry kinetics Mixed inhibitor km and vmax
Mixed inhibitor km and vmax Difference between zero and first order kinetics
Difference between zero and first order kinetics Kinetics reaction
Kinetics reaction Dynafit software
Dynafit software Kinetics of rigid bodies
Kinetics of rigid bodies Kinetics and equilibrium
Kinetics and equilibrium Data kinetics ltd
Data kinetics ltd Pseudo first order equation
Pseudo first order equation Kinetics of crystal violet fading
Kinetics of crystal violet fading Metaboloism
Metaboloism Vampnets for deep learning of molecular kinetics
Vampnets for deep learning of molecular kinetics Growth kinetics
Growth kinetics Enzyme kinetics
Enzyme kinetics Motion of rigid body in three dimensions
Motion of rigid body in three dimensions Factors affecting distribution of drugs
Factors affecting distribution of drugs Kcat
Kcat Kinetics of particles newton's second law
Kinetics of particles newton's second law Octet kinetics
Octet kinetics Collision theory of kinetics
Collision theory of kinetics Kinetics is the branch of:
Kinetics is the branch of: Myeloblast
Myeloblast Kinetics of a particle: force and acceleration
Kinetics of a particle: force and acceleration Kinetics of rigid bodies
Kinetics of rigid bodies Planar kinetics of a rigid body work and energy
Planar kinetics of a rigid body work and energy Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Kinetics flotation chemicals
Kinetics flotation chemicals Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Are kc and kp equal
Are kc and kp equal Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 1 chemical changes
Section 1 chemical changes Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions 7-1 practice problems chemistry answers
7-1 practice problems chemistry answers Oa carpool
Oa carpool Interest rates
Interest rates Foreign exchange worksheet
Foreign exchange worksheet New york midwives
New york midwives Market based forecasting of exchange rates
Market based forecasting of exchange rates Reaction rate
Reaction rate Iv push formula
Iv push formula Exchange rates lesson
Exchange rates lesson Unit 2 lesson 12 interest rates
Unit 2 lesson 12 interest rates Unit 5 macroeconomics lesson 2 activity 45
Unit 5 macroeconomics lesson 2 activity 45 Txdot average low bid
Txdot average low bid Related rates intro
Related rates intro Relative rates of growth
Relative rates of growth Determinants of interest rates
Determinants of interest rates Walmart pto chart 2021 hourly
Walmart pto chart 2021 hourly Option adjusted spread
Option adjusted spread 6-2 pay periods and hourly rates
6-2 pay periods and hourly rates Gcu life insurance
Gcu life insurance Ratios rates and proportions
Ratios rates and proportions Balancing act chemistry
Balancing act chemistry Gjgny
Gjgny Interest rates
Interest rates Chapter 18 reaction rates and equilibrium answer key
Chapter 18 reaction rates and equilibrium answer key 1popularity
1popularity