Spectrochemical Series Cr 3 Safety Aqueous Cr 3
- Slides: 9

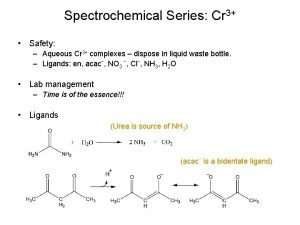
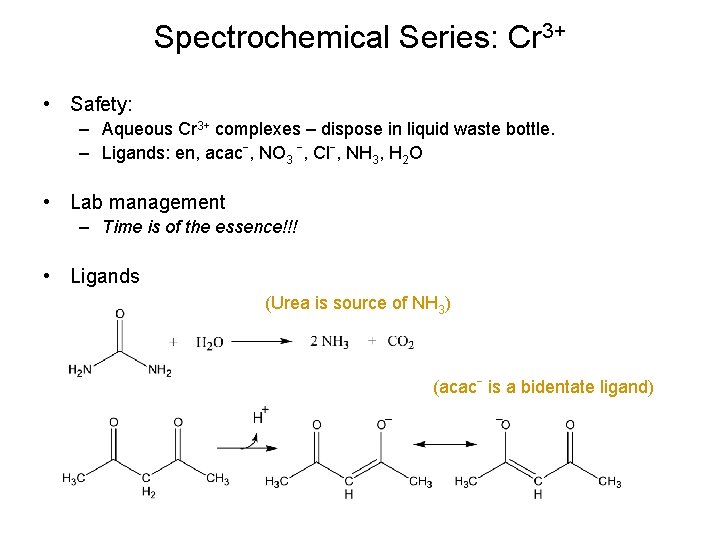
Spectrochemical Series: Cr 3+ • Safety: – Aqueous Cr 3+ complexes – dispose in liquid waste bottle. – Ligands: en, acacˉ, NO 3 ˉ, Clˉ, NH 3, H 2 O • Lab management – Time is of the essence!!! • Ligands (Urea is source of NH 3) (acacˉ is a bidentate ligand)

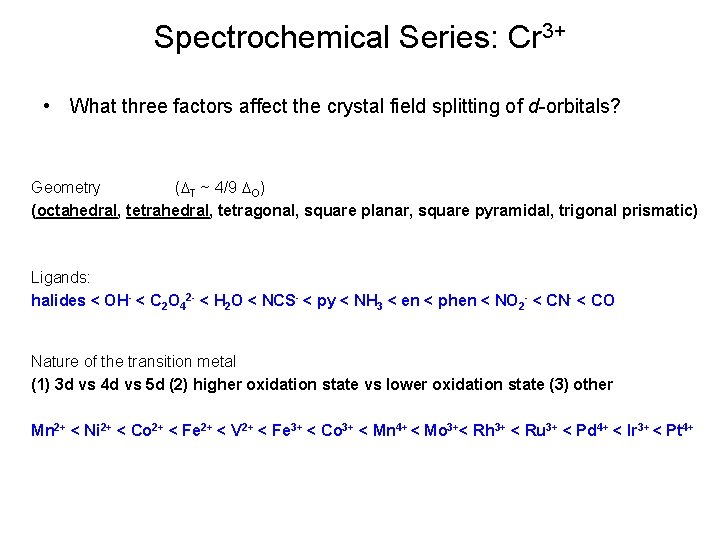
Spectrochemical Series: Cr 3+ • What three factors affect the crystal field splitting of d-orbitals? Geometry (DT ~ 4/9 DO) (octahedral, tetragonal, square planar, square pyramidal, trigonal prismatic) Ligands: halides < OH- < C 2 O 42 - < H 2 O < NCS- < py < NH 3 < en < phen < NO 2 - < CN- < CO Nature of the transition metal (1) 3 d vs 4 d vs 5 d (2) higher oxidation state vs lower oxidation state (3) other Mn 2+ < Ni 2+ < Co 2+ < Fe 2+ < V 2+ < Fe 3+ < Co 3+ < Mn 4+ < Mo 3+< Rh 3+ < Ru 3+ < Pd 4+ < Ir 3+ < Pt 4+

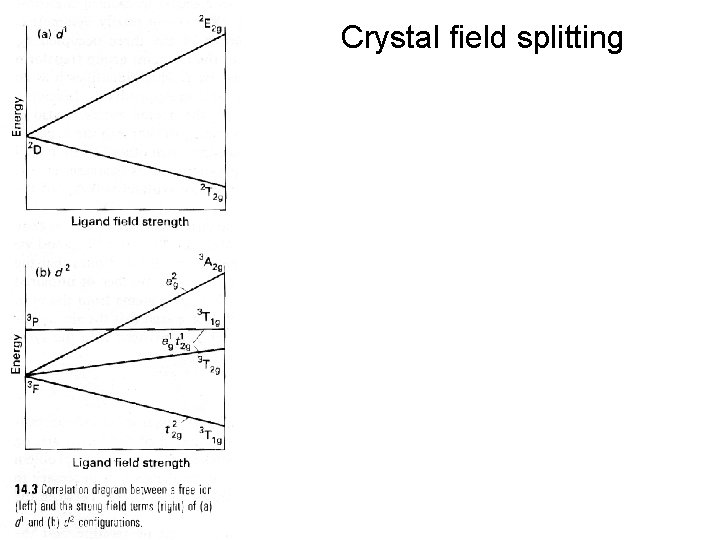
Crystal field splitting

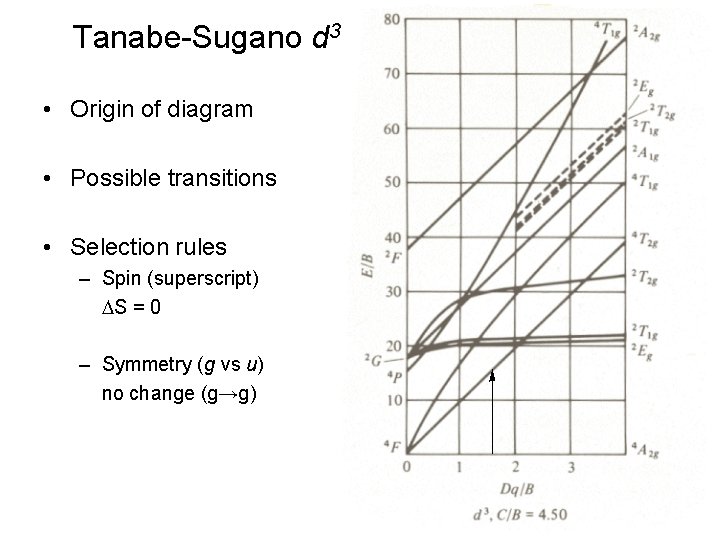
Tanabe-Sugano d 3 • Origin of diagram • Possible transitions • Selection rules – Spin (superscript) DS = 0 – Symmetry (g vs u) no change (g→g)

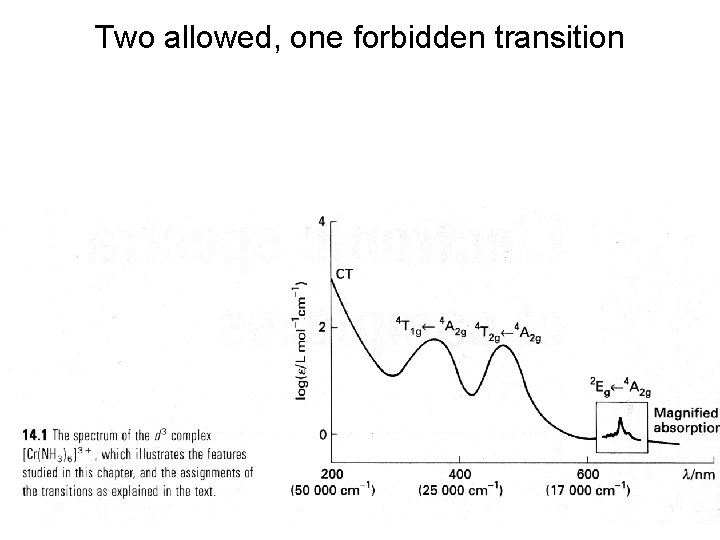
Two allowed, one forbidden transition

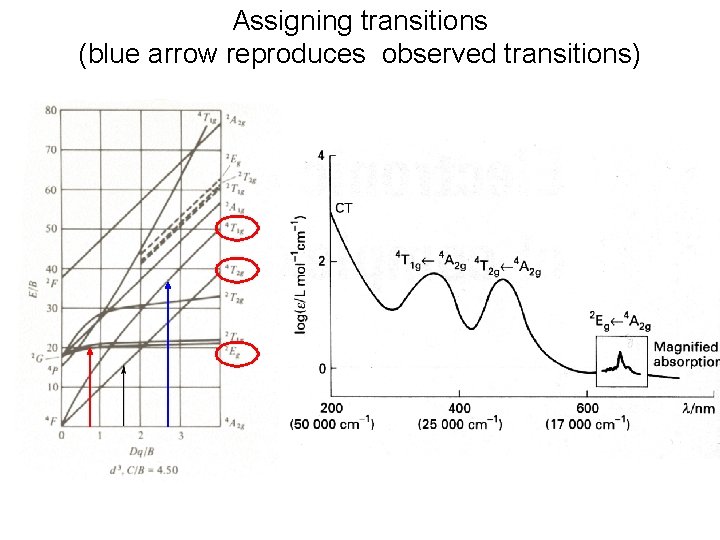
Assigning transitions (blue arrow reproduces observed transitions)

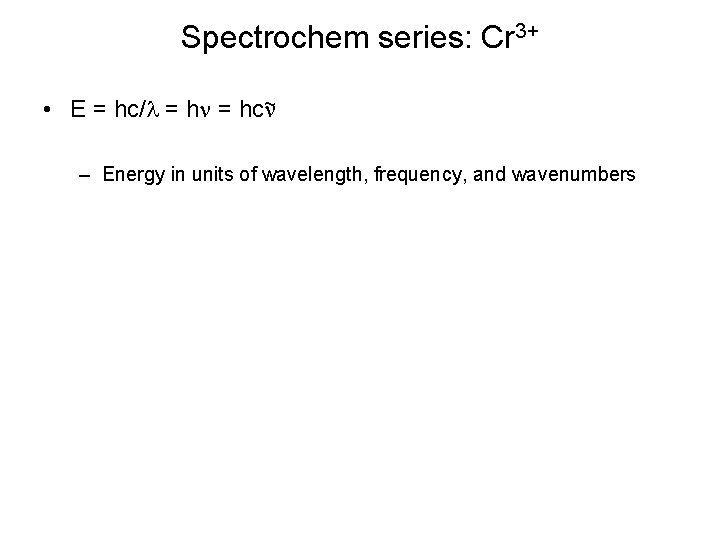
Spectrochem series: Cr 3+ • E = hc/l = hn = hcn~ – Energy in units of wavelength, frequency, and wavenumbers

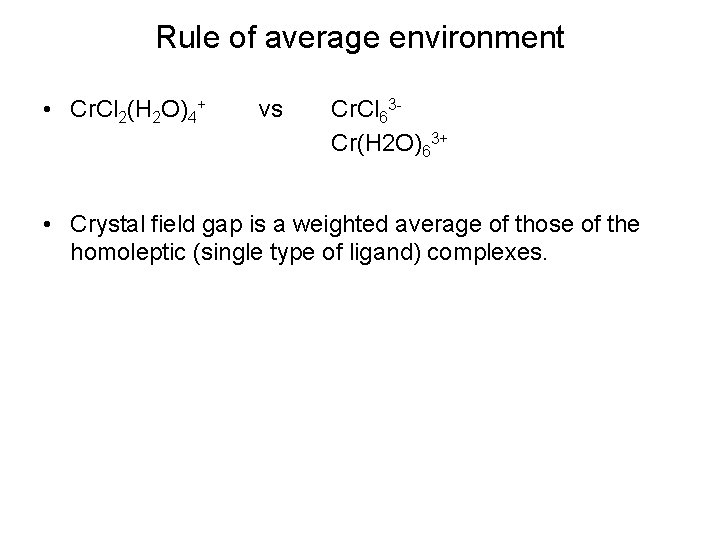
Rule of average environment • Cr. Cl 2(H 2 O)4+ vs Cr. Cl 63 Cr(H 2 O)63+ • Crystal field gap is a weighted average of those of the homoleptic (single type of ligand) complexes.

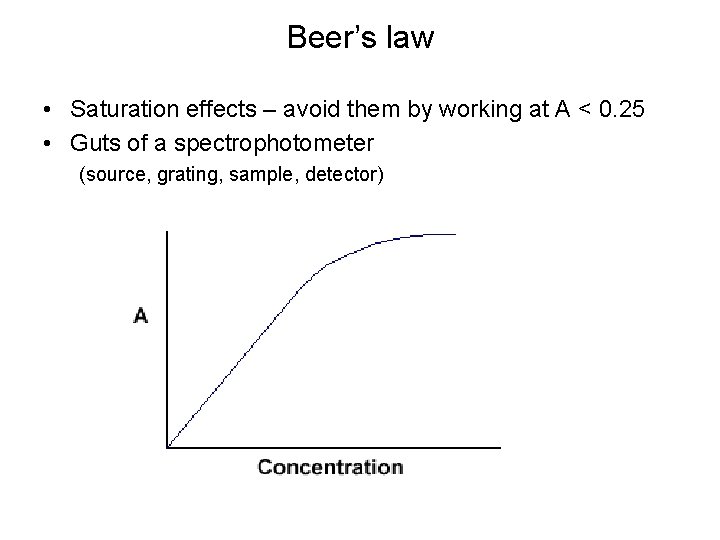
Beer’s law • Saturation effects – avoid them by working at A < 0. 25 • Guts of a spectrophotometer (source, grating, sample, detector)