SOLID STATE AND SURFACE CHEMISTRY LECTURE 2 ADSORPTION

![1/θ = 1+ 1/ Kc [A] At very high concentrations 1 -θ = 1 1/θ = 1+ 1/ Kc [A] At very high concentrations 1 -θ = 1](https://slidetodoc.com/presentation_image_h/9fd76e232fa68032e7d9838a445bf2a0/image-5.jpg)
![For the adsorption of gases on solids the concentration of the gas [A] For the adsorption of gases on solids the concentration of the gas [A]](https://slidetodoc.com/presentation_image_h/9fd76e232fa68032e7d9838a445bf2a0/image-6.jpg)
![EXAMPLE (2) Kd Kc = Ka/Kd = [A—S]2/[A 2][S]2 Rate of desorption = υd EXAMPLE (2) Kd Kc = Ka/Kd = [A—S]2/[A 2][S]2 Rate of desorption = υd](https://slidetodoc.com/presentation_image_h/9fd76e232fa68032e7d9838a445bf2a0/image-10.jpg)
![1/θ= 1 + 1/kc ½ [A 2 ] ½ For the adsorption of gases 1/θ= 1 + 1/kc ½ [A 2 ] ½ For the adsorption of gases](https://slidetodoc.com/presentation_image_h/9fd76e232fa68032e7d9838a445bf2a0/image-11.jpg)
- Slides: 11

SOLID STATE AND SURFACE CHEMISTRY (LECTURE 2 ADSORPTION) Dr. Saeda Rwede Al-Mhyawi Assistant professor in physical chemistry

OBJECTIVES By the end of this section you should: Be able to drive Langmuir isotherm Know the different form of Langmuir equation Know the physical meaning of Langmuir isotherm Dr. saeda Al-Mhyawi

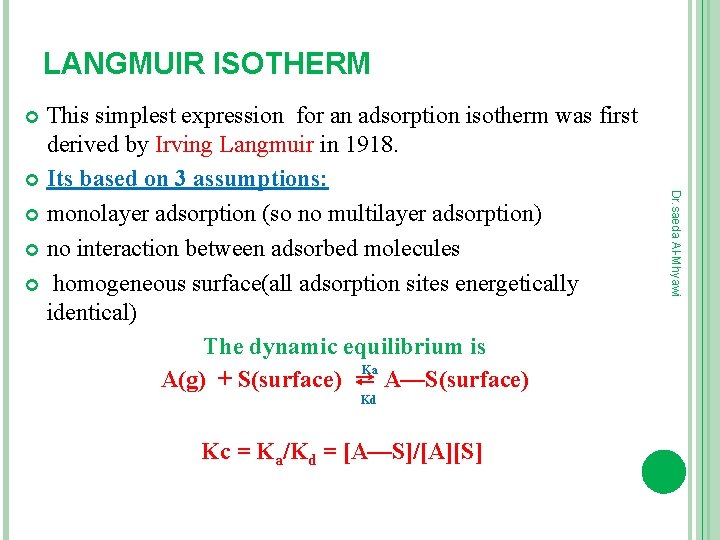
LANGMUIR ISOTHERM This simplest expression for an adsorption isotherm was first derived by Irving Langmuir in 1918. Its based on 3 assumptions: monolayer adsorption (so no multilayer adsorption) no interaction between adsorbed molecules homogeneous surface(all adsorption sites energetically identical) The dynamic equilibrium is Ka A(g) + S(surface) ⇄ A—S(surface) Kc = Ka/Kd = [A—S]/[A][S] Dr. saeda Al-Mhyawi Kd

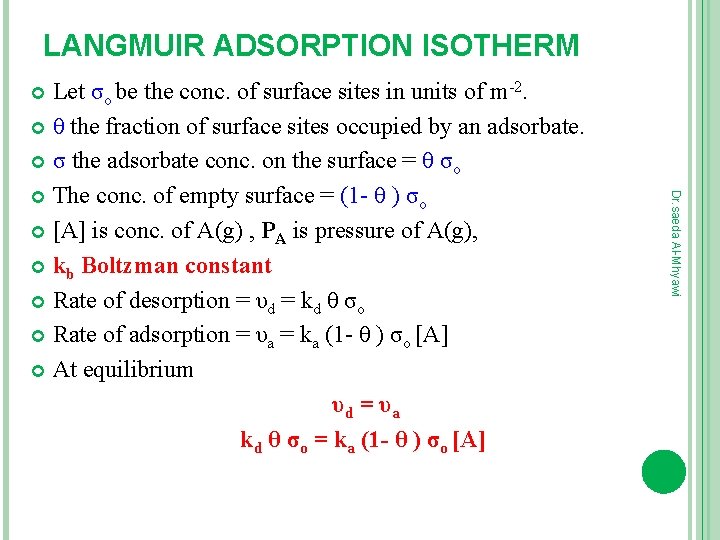
LANGMUIR ADSORPTION ISOTHERM Let σо be the conc. of surface sites in units of m-2. θ the fraction of surface sites occupied by an adsorbate. σ the adsorbate conc. on the surface = θ σо The conc. of empty surface = (1 - θ ) σо [A] is conc. of A(g) , PA is pressure of A(g), kb Boltzman constant Rate of desorption = υd = kd θ σо Rate of adsorption = υa = ka (1 - θ ) σо [A] At equilibrium υd = υa kd θ σо = ka (1 - θ ) σо [A] Dr. saeda Al-Mhyawi
![1θ 1 1 Kc A At very high concentrations 1 θ 1 1/θ = 1+ 1/ Kc [A] At very high concentrations 1 -θ = 1](https://slidetodoc.com/presentation_image_h/9fd76e232fa68032e7d9838a445bf2a0/image-5.jpg)
1/θ = 1+ 1/ Kc [A] At very high concentrations 1 -θ = 1 / Kc [A] i. e. The surface becomes saturated with molecules at high pressures. Dr. saeda Al-Mhyawi θ σо = ka/ kd (1 - θ ) σо [A] Kc= ka/kd θ = Kc [A] - θ Kc [A] θ + θ Kc [A] = Kc [A] θ (1+ Kc [A]) = Kc [A] θ = Kc [A]/ (1+ Kc [A]) 1/θ = 1/ Kc [A]/ + Kc [A]/ Kc [A]
![For the adsorption of gases on solids the concentration of the gas A For the adsorption of gases on solids the concentration of the gas [A]](https://slidetodoc.com/presentation_image_h/9fd76e232fa68032e7d9838a445bf2a0/image-6.jpg)
For the adsorption of gases on solids the concentration of the gas [A] can be replaced by the partial pressure of the gas P. So, the ideal gas law can be used, then [A] = PA/k. BT If we define b = Kc/ KB T, Equation (6 ) becomes: ( kb is Boltzmann constant) Let vm is the amount that can be adsorbed at monolayer coverage and v the amount adsorbed at any pressure (for a unit mass of adsorbent) then: θ = v / vm we can rewrite the last two equations as: 1/v = 1/ Pbvm +1/vm From the equation, we see that a plot of 1/v versus 1/p will have a slope of 1/bvm and an intercept of 1/vm. Dr. saeda Al-Mhyawi 1/ θ = 1+ 1/b. PA

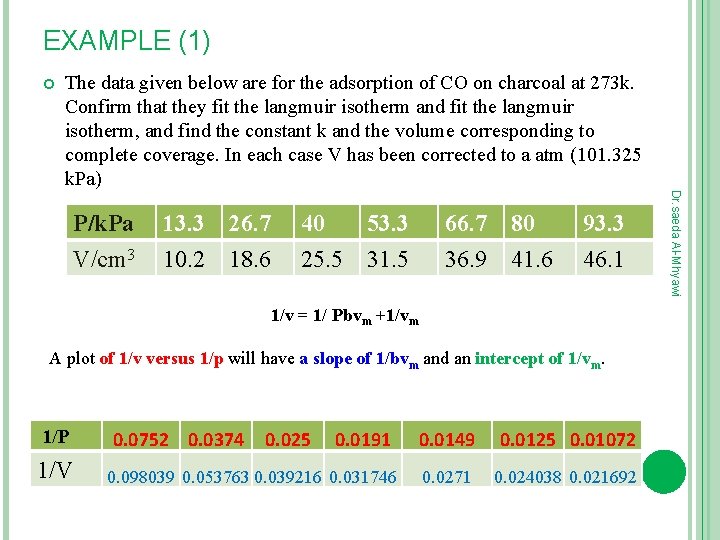
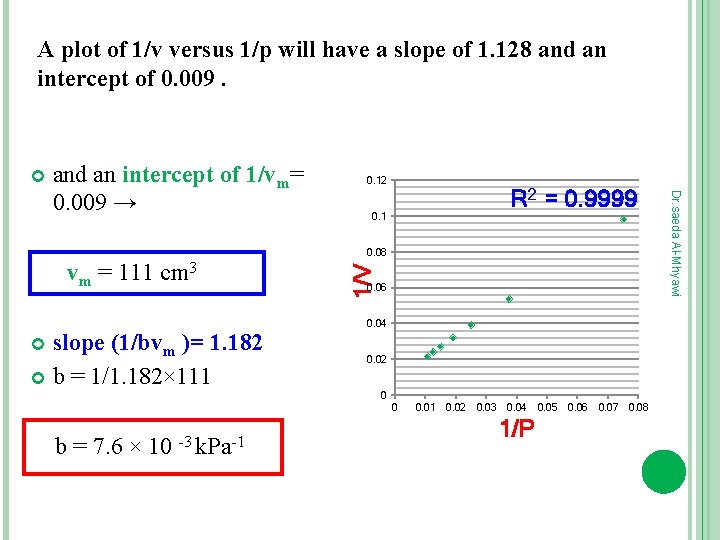
EXAMPLE (1) The data given below are for the adsorption of CO on charcoal at 273 k. Confirm that they fit the langmuir isotherm and fit the langmuir isotherm, and find the constant k and the volume corresponding to complete coverage. In each case V has been corrected to a atm (101. 325 k. Pa) 13. 3 10. 2 26. 7 18. 6 40 25. 5 53. 3 31. 5 66. 7 36. 9 80 41. 6 93. 3 46. 1 1/v = 1/ Pbvm +1/vm A plot of 1/v versus 1/p will have a slope of 1/bvm and an intercept of 1/vm. 1/P 1/V 0. 0752 0. 0374 0. 025 0. 0191 0. 098039 0. 053763 0. 039216 0. 031746 0. 0149 0. 0125 0. 01072 0. 0271 0. 024038 0. 021692 Dr. saeda Al-Mhyawi P/k. Pa V/cm 3

A plot of 1/v versus 1/p will have a slope of 1. 128 and an intercept of 0. 009. 0. 12 R 2 = 0. 9999 0. 1 vm = 111 cm 3 slope (1/bvm )= 1. 182 b = 1/1. 182× 111 1/V 0. 08 0. 06 0. 04 0. 02 0 0 b = 7. 6 × 10 -3 k. Pa-1 0. 02 0. 03 0. 04 1/P 0. 05 0. 06 0. 07 0. 08 Dr. saeda Al-Mhyawi and an intercept of 1/vm= 0. 009 →

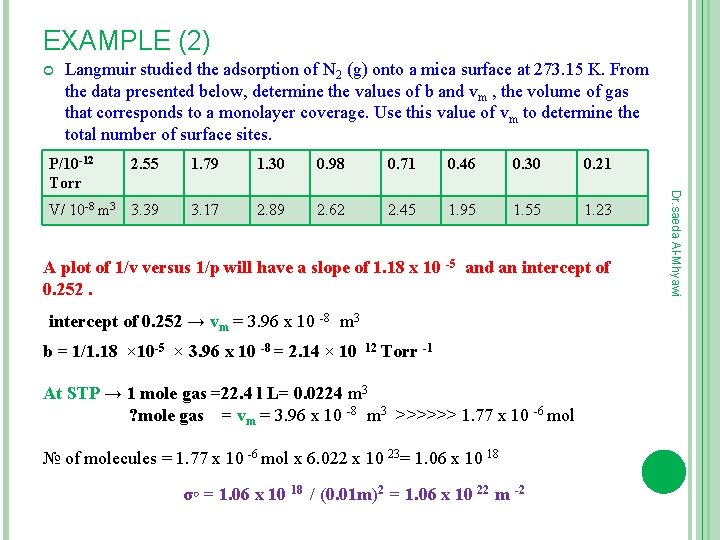
EXAMPLE (2) Langmuir studied the adsorption of N 2 (g) onto a mica surface at 273. 15 K. From the data presented below, determine the values of b and vm , the volume of gas that corresponds to a monolayer coverage. Use this value of vm to determine the total number of surface sites. 2. 55 1. 79 1. 30 0. 98 0. 71 0. 46 0. 30 0. 21 V/ 10 -8 m 3 3. 39 3. 17 2. 89 2. 62 2. 45 1. 95 1. 55 1. 23 A plot of 1/v versus 1/p will have a slope of 1. 18 x 10 -5 and an intercept of 0. 252 → vm = 3. 96 x 10 -8 m 3 b = 1/1. 18 × 10 -5 × 3. 96 x 10 -8 = 2. 14 × 10 12 Torr -1 At STP → 1 mole gas =22. 4 l L= 0. 0224 m 3 ? mole gas = vm = 3. 96 x 10 -8 m 3 >>>>>> 1. 77 x 10 -6 mol № of molecules = 1. 77 x 10 -6 mol x 6. 022 x 10 23= 1. 06 x 10 18 σ◦ = 1. 06 x 10 18 / (0. 01 m)2 = 1. 06 x 10 22 m -2 Dr. saeda Al-Mhyawi P/10 -12 Torr
![EXAMPLE 2 Kd Kc KaKd AS2A 2S2 Rate of desorption υd EXAMPLE (2) Kd Kc = Ka/Kd = [A—S]2/[A 2][S]2 Rate of desorption = υd](https://slidetodoc.com/presentation_image_h/9fd76e232fa68032e7d9838a445bf2a0/image-10.jpg)
EXAMPLE (2) Kd Kc = Ka/Kd = [A—S]2/[A 2][S]2 Rate of desorption = υd = kd θ 2 σо 2 Rate of adsorption = υa = ka (1 - θ )2 σо 2[A 2] At equilibrium υd = υa kd θ σо 2 = ka (1 - θ )2 σо 2[A 2] θ 2 = ka (1 - θ )2 [A 2]/ kd Dr. saeda Al-Mhyawi Derive the Langmuir adsorption isotherm for the case in which a diatomic molecule dissociates upon adsorption to the surface. Ka A 2(g) + S(surface) ⇄ A—S(surface)
![1θ 1 1kc ½ A 2 ½ For the adsorption of gases 1/θ= 1 + 1/kc ½ [A 2 ] ½ For the adsorption of gases](https://slidetodoc.com/presentation_image_h/9fd76e232fa68032e7d9838a445bf2a0/image-11.jpg)
1/θ= 1 + 1/kc ½ [A 2 ] ½ For the adsorption of gases on solids the concentration of the gas [A] can be replaced by the partial pressure of the gas P. 1/θ= 1 + 1/b A 2 ½ PA 2 ½ Dr. saeda Al-Mhyawi θ 2 = kc (1 - θ )2 [A 2 ] θ = kc ½ [A 2 ] ½ - kc ½ [A 2 ] ½ θ θ + kc ½ [A 2 ] ½ θ = kc ½ [A 2 ] ½ θ(1 + kc ½ [A 2 ] ½ ) = kc ½ [A 2 ] ½ θ= kc ½ [A 2 ] ½ /(1 + kc ½ [A 2 ] ½ )