SixMonth IVUS and 12 Month Clinical Outcomes in










- Slides: 28

Six-Month IVUS and 12 -Month Clinical Outcomes in the EVOLVE FHU Trial: A Randomized Evaluation of a Novel Bioabsorbable Polymer-Coated, Everolimus-Eluting Stent Stefan Verheye, Ian T. Meredith, Neil J. Weissman, Paul Barragan, Douglas Scott, Mariano Valdés Chavarri, Nick E. J. West, Henning Kelbaek, Robert Whitbourn, Daren Walters, Jacek Kubica, Dominic J. Allocco, Keith D. Dawkins Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Meredith ● EVOLVE overview ● TCT 2010 ● Washington, DC Slide 1

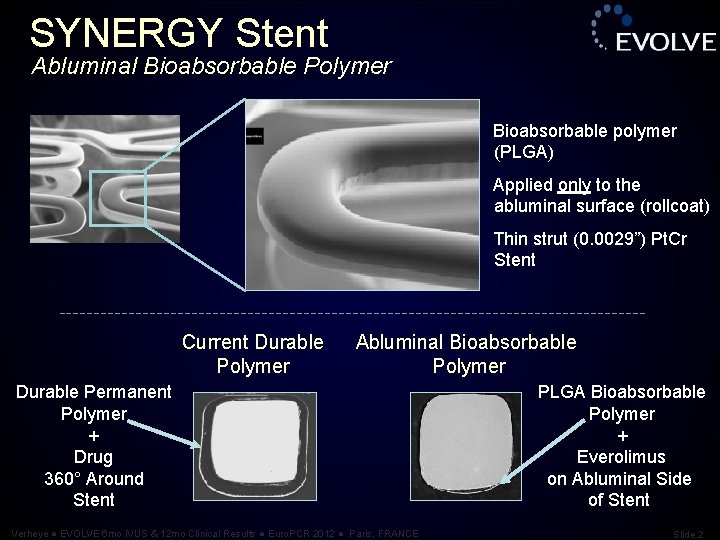
SYNERGY Stent Abluminal Bioabsorbable Polymer Bioabsorbable polymer (PLGA) Applied only to the abluminal surface (rollcoat) Thin strut (0. 0029”) Pt. Cr Stent Current Durable Polymer Abluminal Bioabsorbable Polymer Durable Permanent Polymer + Drug 360° Around Stent Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE PLGA Bioabsorbable Polymer + Everolimus on Abluminal Side of Stent Slide 2

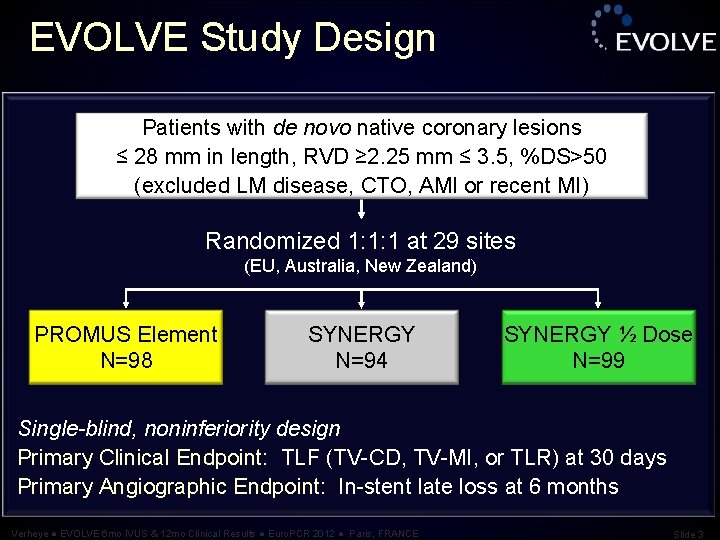
EVOLVE Study Design Patients with de novo native coronary lesions ≤ 28 mm in length, RVD ≥ 2. 25 mm ≤ 3. 5, %DS>50 (excluded LM disease, CTO, AMI or recent MI) Randomized 1: 1: 1 at 29 sites (EU, Australia, New Zealand) PROMUS Element N=98 SYNERGY N=94 SYNERGY ½ Dose N=99 Single-blind, noninferiority design Primary Clinical Endpoint: TLF (TV-CD, TV-MI, or TLR) at 30 days Primary Angiographic Endpoint: In-stent late loss at 6 months Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 3

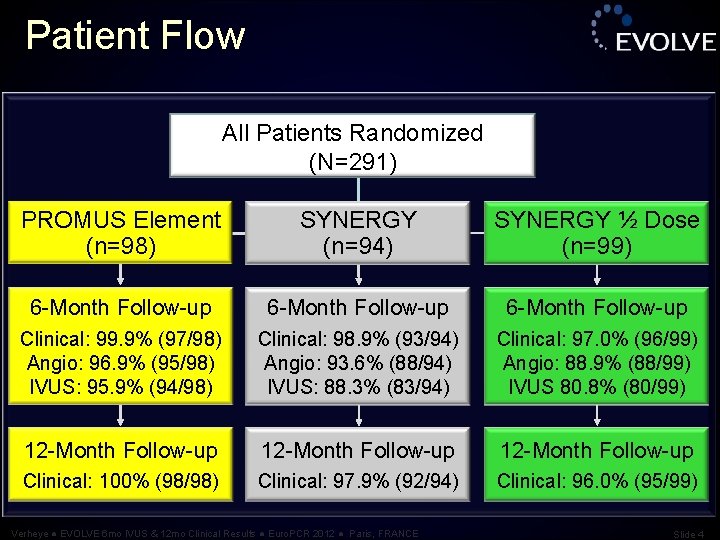
Patient Flow All Patients Randomized (N=291) PROMUS Element (n=98) SYNERGY (n=94) SYNERGY ½ Dose (n=99) 6 -Month Follow-up Clinical: 99. 9% (97/98) Angio: 96. 9% (95/98) IVUS: 95. 9% (94/98) Clinical: 98. 9% (93/94) Angio: 93. 6% (88/94) IVUS: 88. 3% (83/94) Clinical: 97. 0% (96/99) Angio: 88. 9% (88/99) IVUS 80. 8% (80/99) 12 -Month Follow-up Clinical: 100% (98/98) Clinical: 97. 9% (92/94) Clinical: 96. 0% (95/99) Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 4

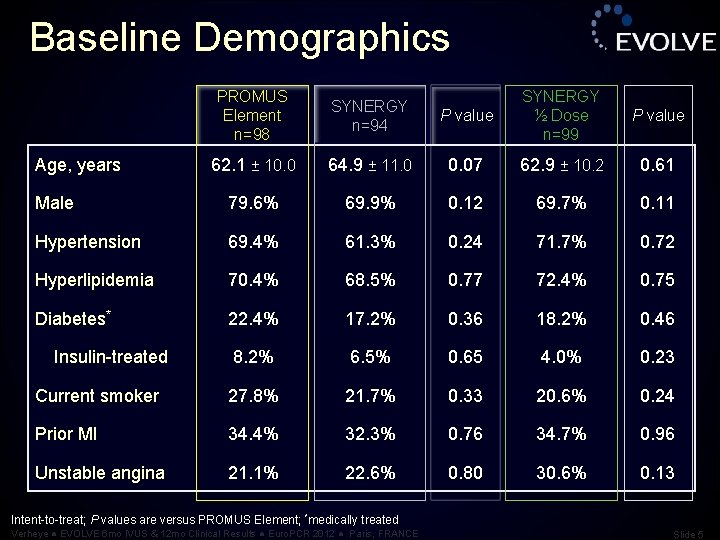
Baseline Demographics PROMUS Element n=98 SYNERGY n=94 P value SYNERGY ½ Dose n=99 P value 62. 1 ± 10. 0 64. 9 ± 11. 0 0. 07 62. 9 ± 10. 2 0. 61 Male 79. 6% 69. 9% 0. 12 69. 7% 0. 11 Hypertension 69. 4% 61. 3% 0. 24 71. 7% 0. 72 Hyperlipidemia 70. 4% 68. 5% 0. 77 72. 4% 0. 75 Diabetes* 22. 4% 17. 2% 0. 36 18. 2% 0. 46 8. 2% 6. 5% 0. 65 4. 0% 0. 23 Current smoker 27. 8% 21. 7% 0. 33 20. 6% 0. 24 Prior MI 34. 4% 32. 3% 0. 76 34. 7% 0. 96 Unstable angina 21. 1% 22. 6% 0. 80 30. 6% 0. 13 Age, years Insulin-treated Intent-to-treat; P values are versus PROMUS Element; *medically treated Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 5

Baseline Lesion Characteristics PROMUS Element n=98 SYNERGY n=94 P value SYNERGY ½ Dose n=99 P value RVD, mm 2. 53 ± 0. 41 2. 60 ± 0. 45 0. 22 2. 65 ± 0. 40 0. 04 MLD, mm 0. 68 ± 0. 30 0. 96 0. 67 ± 0. 31 0. 94 DS, % 73. 4% ± 9. 9 74. 0% ± 10. 4 0. 68 74. 7% ± 10. 5 0. 35 Lesion length, mm 14. 62 ± 5. 81 13. 41 ± 6. 29 0. 16 13. 55 ± 5. 76 0. 21 LAD 39. 8% 41. 8% 0. 78 39. 4% 0. 95 LCx 31. 6% 26. 4% 0. 43 33. 3% 0. 80 RCA 28. 6% 31. 9% 0. 62 27. 3% 0. 84 Lesion location Lesion characteristics evaluated by QCA. Intent-to-treat; P values are versus PROMUS Element Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 6

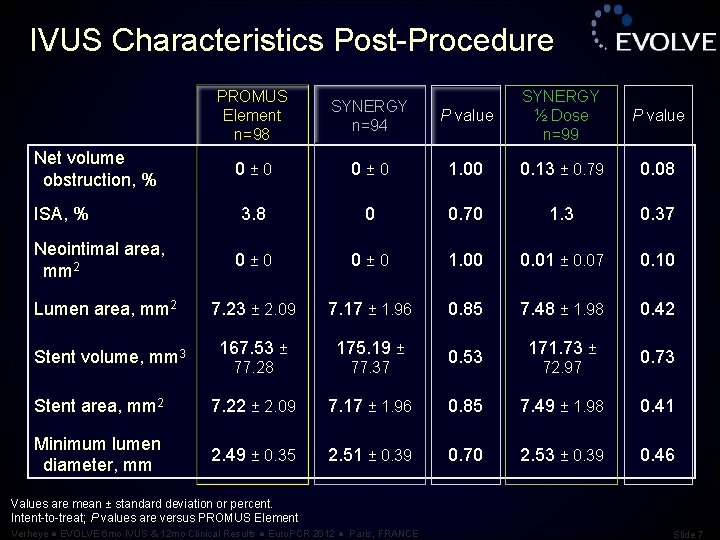
IVUS Characteristics Post-Procedure P value SYNERGY ½ Dose n=99 P value 0 ± 0 1. 00 0. 13 ± 0. 79 0. 08 3. 8 0 0. 70 1. 3 0. 37 Neointimal area, mm 2 0 ± 0 1. 00 0. 01 ± 0. 07 0. 10 Lumen area, mm 2 7. 23 ± 2. 09 7. 17 ± 1. 96 0. 85 7. 48 ± 1. 98 0. 42 167. 53 ± 175. 19 ± 77. 28 77. 37 Stent area, mm 2 7. 22 ± 2. 09 7. 17 ± 1. 96 0. 85 7. 49 ± 1. 98 0. 41 Minimum lumen diameter, mm 2. 49 ± 0. 35 2. 51 ± 0. 39 0. 70 2. 53 ± 0. 39 0. 46 Net volume obstruction, % ISA, % Stent volume, mm 3 PROMUS Element n=98 SYNERGY n=94 0 ± 0 0. 53 171. 73 ± 72. 97 0. 73 Values are mean ± standard deviation or percent. Intent-to-treat; P values are versus PROMUS Element Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 7

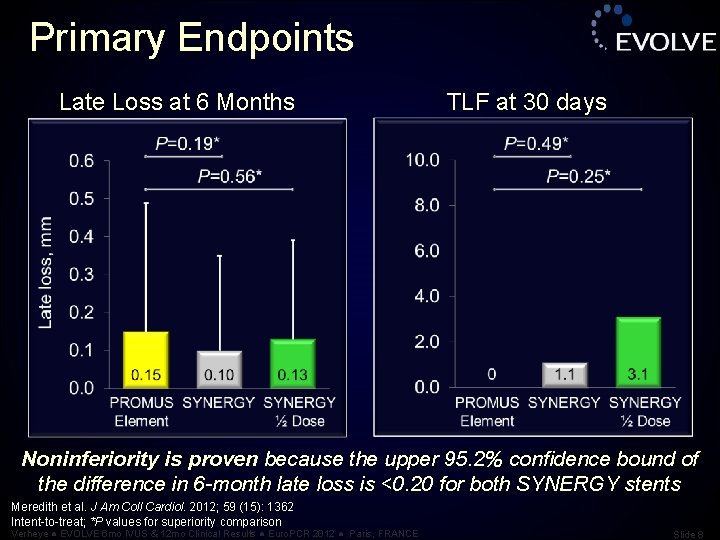
Primary Endpoints Late Loss at 6 Months TLF at 30 days Noninferiority is proven because the upper 95. 2% confidence bound of the difference in 6 -month late loss is <0. 20 for both SYNERGY stents Meredith et al. J Am Coll Cardiol. 2012; 59 (15): 1362 Intent-to-treat; *P values for superiority comparison Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 8

ISA at 6 Months Intent-to-treat, paired data Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 9

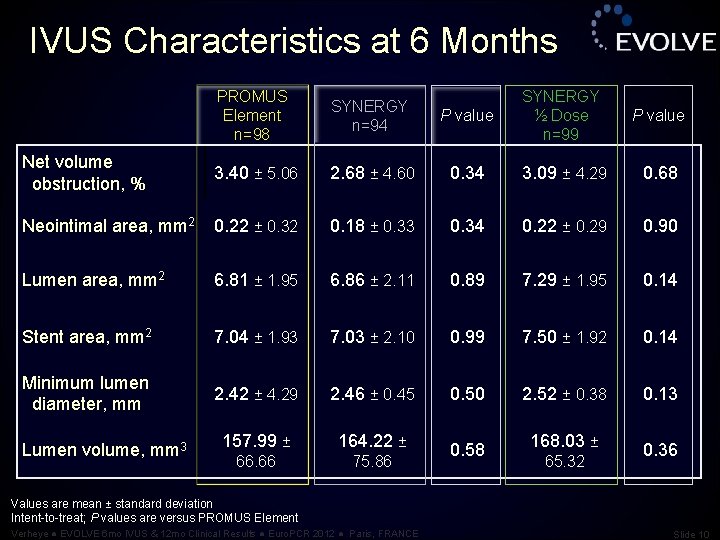
IVUS Characteristics at 6 Months PROMUS Element n=98 SYNERGY n=94 P value SYNERGY ½ Dose n=99 P value Net volume obstruction, % 3. 40 ± 5. 06 2. 68 ± 4. 60 0. 34 3. 09 ± 4. 29 0. 68 Neointimal area, mm 2 0. 22 ± 0. 32 0. 18 ± 0. 33 0. 34 0. 22 ± 0. 29 0. 90 Lumen area, mm 2 6. 81 ± 1. 95 6. 86 ± 2. 11 0. 89 7. 29 ± 1. 95 0. 14 Stent area, mm 2 7. 04 ± 1. 93 7. 03 ± 2. 10 0. 99 7. 50 ± 1. 92 0. 14 Minimum lumen diameter, mm 2. 42 ± 4. 29 2. 46 ± 0. 45 0. 50 2. 52 ± 0. 38 0. 13 157. 99 ± 164. 22 ± 66. 66 75. 86 Lumen volume, mm 3 0. 58 168. 03 ± 65. 32 0. 36 Values are mean ± standard deviation Intent-to-treat; P values are versus PROMUS Element Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 10

Death and MI at 12 Months One NQWMI in the SYNERGY group and all 3 NQWMI in the SYNERGY ½ Dose group were periprocedural. The remaining 2 NQWMI in the SYNERGY arm were considered unrelated to study device: one at day 347 due to anemia and one at day 364 subsequent to respiratory failure in a patient with severe COPD. Intent-to-treat; P values are versus PROMUS Element (Fisher exact test) Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 11

Revasc and ST at 12 Months Intent-to-treat; P values are versus PROMUS Element (Fisher exact test) Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 12

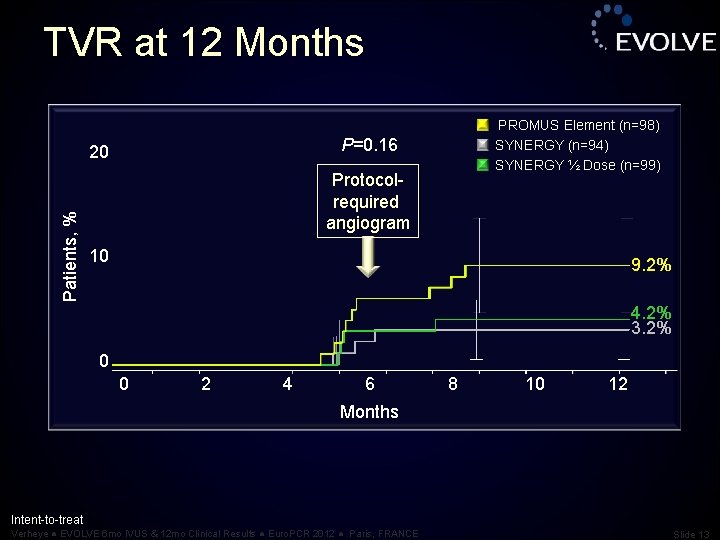
TVR at 12 Months P=0. 16 20 Patients, % PROMUS Element (n=98) SYNERGY (n=94) SYNERGY ½ Dose (n=99) Protocolrequired angiogram 10 9. 2% 4. 2% 3. 2% 0 0 2 4 6 8 10 12 Months Intent-to-treat Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 13

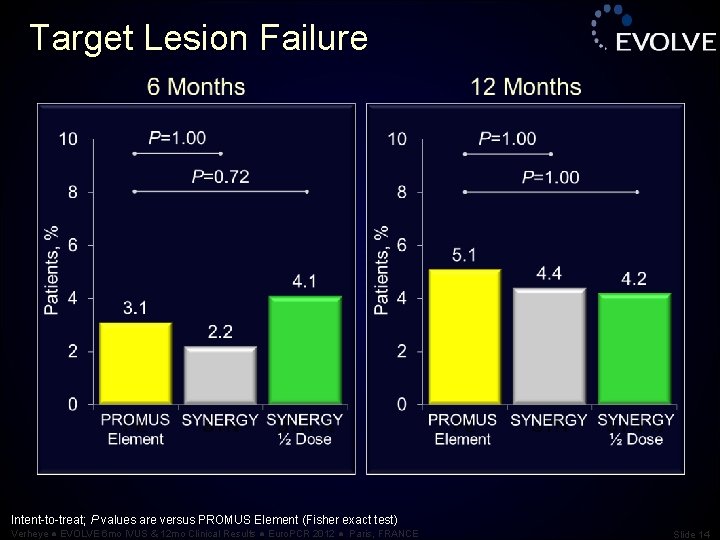
Target Lesion Failure Intent-to-treat; P values are versus PROMUS Element (Fisher exact test) Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 14

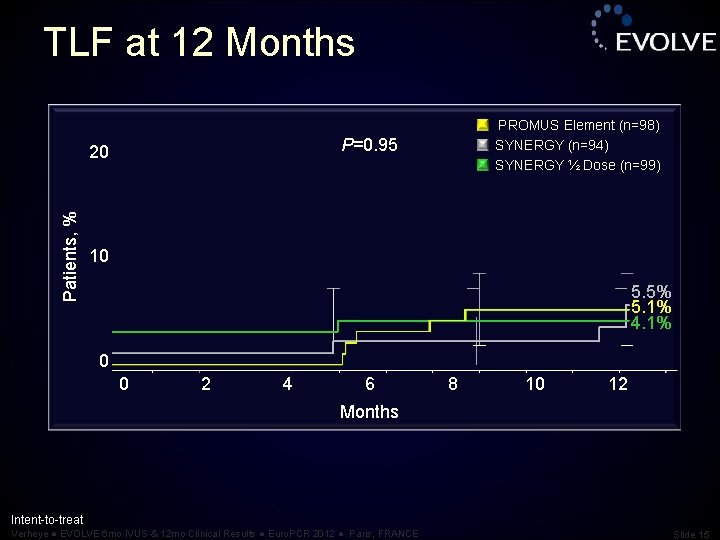
TLF at 12 Months P=0. 95 20 Patients, % PROMUS Element (n=98) SYNERGY (n=94) SYNERGY ½ Dose (n=99) 10 5. 5% 5. 1% 4. 1% 0 0 2 4 6 8 10 12 Months Intent-to-treat Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 15

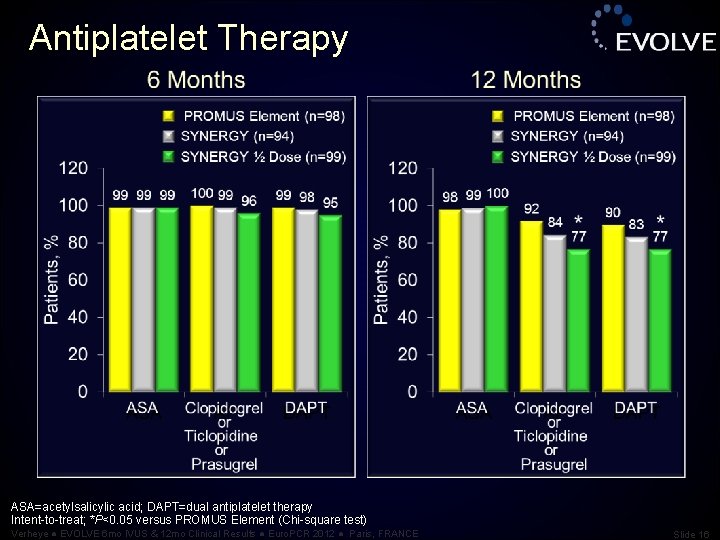
Antiplatelet Therapy ASA=acetylsalicylic acid; DAPT=dual antiplatelet therapy Intent-to-treat; *P<0. 05 versus PROMUS Element (Chi-square test) Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 16

Limitations • The study only included patients with relatively simple de novo lesions – Patients with AMI, stroke, CTO, bifurcation, LMCA lesion, SVG lesion, ostial lesions, or lesions with thrombus or excessive tortuosity or angulation were excluded • The study was not powered to detect differences in clinical event rates • The study was not designed to assess the risk of thrombosis or the required duration of dual antiplatelet therapy with SYNERGY Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 17

Conclusions • At 6 months, everolimus delivered from an ultra-thin bioabsorbable abluminal polymer resulted in equivalent net volume obstruction and ISA compared with a permanent polymer everolimus eluting stent. – No significant differences between groups for any IVUS parameters • At 12 months, no significant differences between groups for clinical endpoints, including TLF, death, MI, revascularization – No incidence of stent thrombosis, cardiac death, or QWMI in any group at 12 months • These data suggest that anti-restenotic efficacy is maintained even with disappearance of the polymer by 120 days • This novel SYNERGY stent may allow shorter or safer interruption of dual antiplatelet therapy post-implantation without compromising inhibition of neointimal growth. Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 18

Backups Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Meredith ● EVOLVE overview ● TCT 2010 ● Washington, DC Slide 19

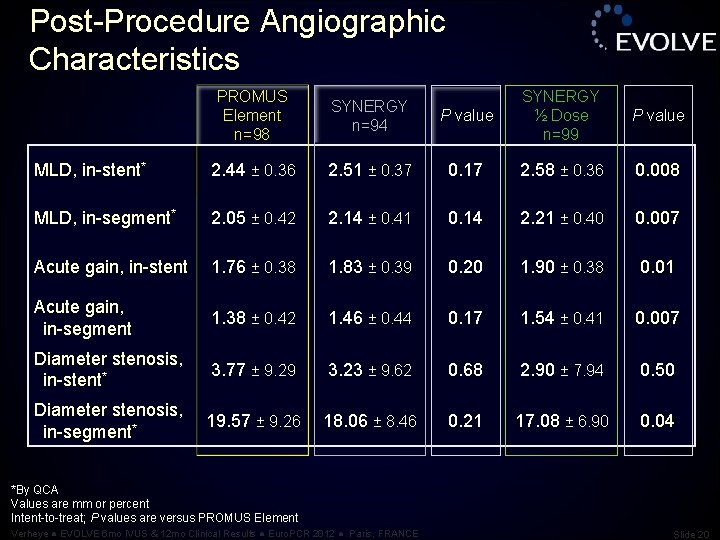
Post-Procedure Angiographic Characteristics PROMUS Element n=98 SYNERGY n=94 P value SYNERGY ½ Dose n=99 P value MLD, in-stent* 2. 44 ± 0. 36 2. 51 ± 0. 37 0. 17 2. 58 ± 0. 36 0. 008 MLD, in-segment* 2. 05 ± 0. 42 2. 14 ± 0. 41 0. 14 2. 21 ± 0. 40 0. 007 Acute gain, in-stent 1. 76 ± 0. 38 1. 83 ± 0. 39 0. 20 1. 90 ± 0. 38 0. 01 Acute gain, in-segment 1. 38 ± 0. 42 1. 46 ± 0. 44 0. 17 1. 54 ± 0. 41 0. 007 Diameter stenosis, in-stent* 3. 77 ± 9. 29 3. 23 ± 9. 62 0. 68 2. 90 ± 7. 94 0. 50 Diameter stenosis, in-segment* 19. 57 ± 9. 26 18. 06 ± 8. 46 0. 21 17. 08 ± 6. 90 0. 04 *By QCA Values are mm or percent Intent-to-treat; P values are versus PROMUS Element Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 20

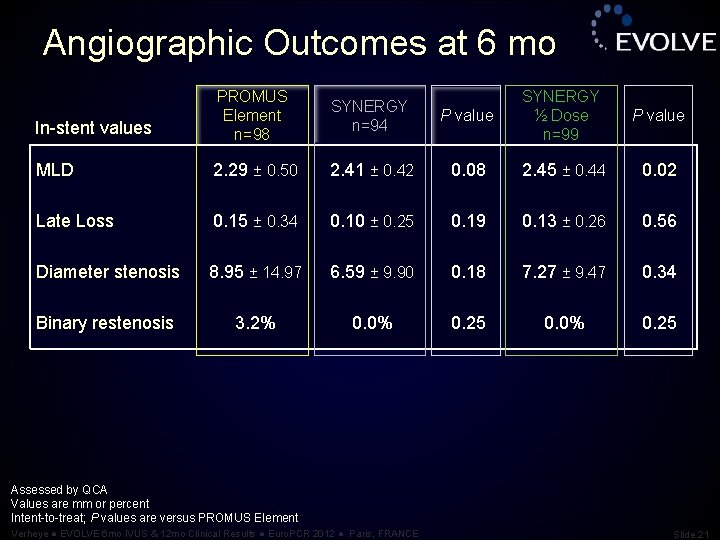
Angiographic Outcomes at 6 mo In-stent values PROMUS Element n=98 SYNERGY n=94 P value SYNERGY ½ Dose n=99 P value MLD 2. 29 ± 0. 50 2. 41 ± 0. 42 0. 08 2. 45 ± 0. 44 0. 02 Late Loss 0. 15 ± 0. 34 0. 10 ± 0. 25 0. 19 0. 13 ± 0. 26 0. 56 Diameter stenosis 8. 95 ± 14. 97 6. 59 ± 9. 90 0. 18 7. 27 ± 9. 47 0. 34 Binary restenosis 3. 2% 0. 0% 0. 25 Assessed by QCA Values are mm or percent Intent-to-treat; P values are versus PROMUS Element Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 21

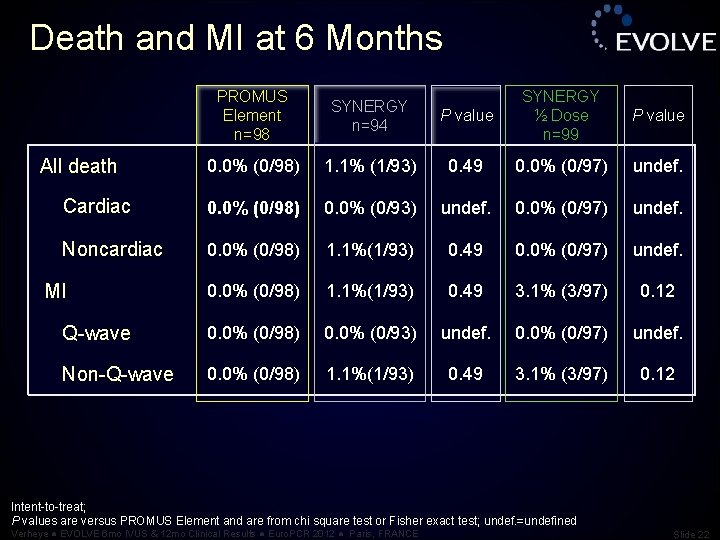
Death and MI at 6 Months PROMUS Element n=98 SYNERGY n=94 P value SYNERGY ½ Dose n=99 P value 0. 0% (0/98) 1. 1% (1/93) 0. 49 0. 0% (0/97) undef. Cardiac 0. 0% (0/98) 0. 0% (0/93) undef. 0. 0% (0/97) undef. Noncardiac 0. 0% (0/98) 1. 1%(1/93) 0. 49 0. 0% (0/97) undef. 0. 0% (0/98) 1. 1%(1/93) 0. 49 3. 1% (3/97) 0. 12 Q-wave 0. 0% (0/98) 0. 0% (0/93) undef. 0. 0% (0/97) undef. Non-Q-wave 0. 0% (0/98) 1. 1%(1/93) 0. 49 3. 1% (3/97) 0. 12 All death MI Intent-to-treat; P values are versus PROMUS Element and are from chi square test or Fisher exact test; undef. =undefined Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 22

Revascularization at 6 Months P value SYNERGY ½ Dose n=99 P value 3. 2% (3/93) 0. 50 2. 1% (2/97) 0. 28 3. 1% (3/98) 1. 1% (1/93) 0. 62 1. 0% (1/97) 0. 62 PCI 3. 1% (3/98) 1. 1% (1/93) 0. 62 1. 0% (1/97) 0. 62 CABG 0. 0% (0/98) 0. 0% (0/93) undef. 0. 0% (0/97) undef. 3. 1% (3/98) 2. 2% (2/93) 1. 00 1. 0% (1/97) 0. 62 TVR TLR Non-TLR TVR PROMUS Element n=98 SYNERGY n=94 6. 1% (6/98) Intent-to-treat; P values are versus PROMUS Element and are from chi square test or Fisher exact test Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 23

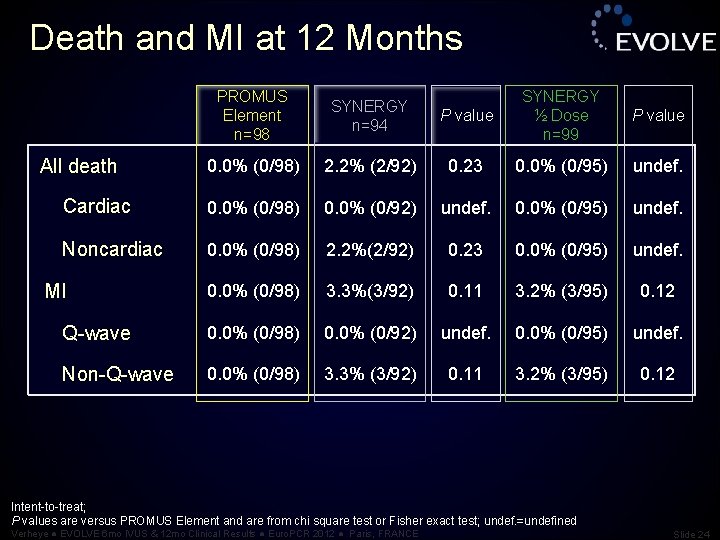
Death and MI at 12 Months PROMUS Element n=98 SYNERGY n=94 P value SYNERGY ½ Dose n=99 P value 0. 0% (0/98) 2. 2% (2/92) 0. 23 0. 0% (0/95) undef. Cardiac 0. 0% (0/98) 0. 0% (0/92) undef. 0. 0% (0/95) undef. Noncardiac 0. 0% (0/98) 2. 2%(2/92) 0. 23 0. 0% (0/95) undef. 0. 0% (0/98) 3. 3%(3/92) 0. 11 3. 2% (3/95) 0. 12 Q-wave 0. 0% (0/98) 0. 0% (0/92) undef. 0. 0% (0/95) undef. Non-Q-wave 0. 0% (0/98) 3. 3% (3/92) 0. 11 3. 2% (3/95) 0. 12 All death MI Intent-to-treat; P values are versus PROMUS Element and are from chi square test or Fisher exact test; undef. =undefined Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 24

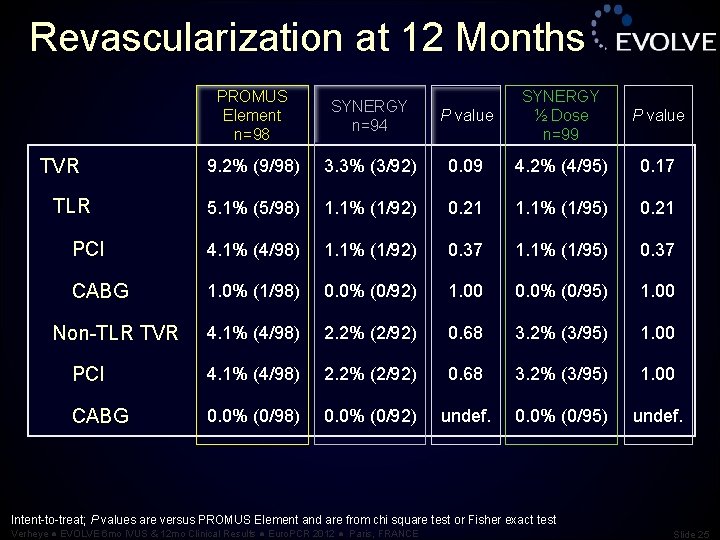
Revascularization at 12 Months P value SYNERGY ½ Dose n=99 P value 3. 3% (3/92) 0. 09 4. 2% (4/95) 0. 17 5. 1% (5/98) 1. 1% (1/92) 0. 21 1. 1% (1/95) 0. 21 PCI 4. 1% (4/98) 1. 1% (1/92) 0. 37 1. 1% (1/95) 0. 37 CABG 1. 0% (1/98) 0. 0% (0/92) 1. 00 0. 0% (0/95) 1. 00 4. 1% (4/98) 2. 2% (2/92) 0. 68 3. 2% (3/95) 1. 00 PCI 4. 1% (4/98) 2. 2% (2/92) 0. 68 3. 2% (3/95) 1. 00 CABG 0. 0% (0/98) 0. 0% (0/92) undef. 0. 0% (0/95) undef. TVR TLR Non-TLR TVR PROMUS Element n=98 SYNERGY n=94 9. 2% (9/98) Intent-to-treat; P values are versus PROMUS Element and are from chi square test or Fisher exact test Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 25

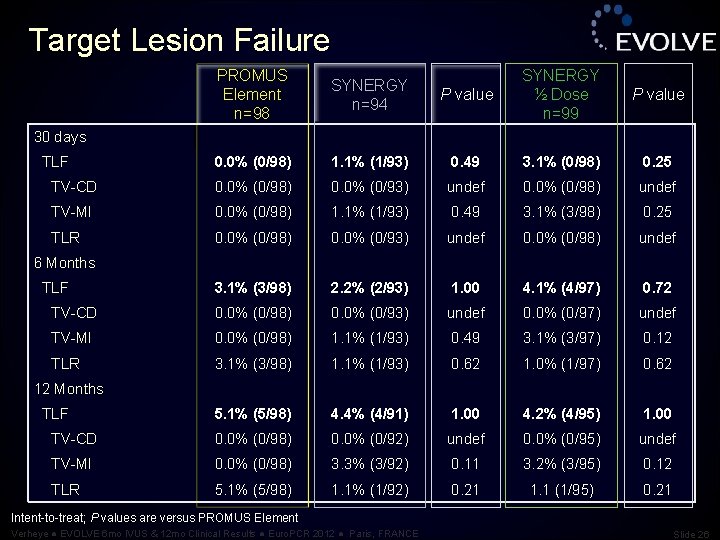
Target Lesion Failure PROMUS Element n=98 SYNERGY n=94 P value SYNERGY ½ Dose n=99 P value 0. 0% (0/98) 1. 1% (1/93) 0. 49 3. 1% (0/98) 0. 25 TV-CD 0. 0% (0/98) 0. 0% (0/93) undef 0. 0% (0/98) undef TV-MI 0. 0% (0/98) 1. 1% (1/93) 0. 49 3. 1% (3/98) 0. 25 TLR 0. 0% (0/98) 0. 0% (0/93) undef 0. 0% (0/98) undef 3. 1% (3/98) 2. 2% (2/93) 1. 00 4. 1% (4/97) 0. 72 TV-CD 0. 0% (0/98) 0. 0% (0/93) undef 0. 0% (0/97) undef TV-MI 0. 0% (0/98) 1. 1% (1/93) 0. 49 3. 1% (3/97) 0. 12 TLR 3. 1% (3/98) 1. 1% (1/93) 0. 62 1. 0% (1/97) 0. 62 5. 1% (5/98) 4. 4% (4/91) 1. 00 4. 2% (4/95) 1. 00 TV-CD 0. 0% (0/98) 0. 0% (0/92) undef 0. 0% (0/95) undef TV-MI 0. 0% (0/98) 3. 3% (3/92) 0. 11 3. 2% (3/95) 0. 12 TLR 5. 1% (5/98) 1. 1% (1/92) 0. 21 1. 1 (1/95) 0. 21 30 days TLF 6 Months TLF 12 Months TLF Intent-to-treat; P values are versus PROMUS Element Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 26

TLR at 12 Months P=0. 14 20 Patients, % PROMUS Element (n=98) SYNERGY (n=94) SYNERGY ½ Dose (n=99) Protocolrequired angiogram 10 5. 1% 1. 0% 0 0 2 4 6 8 10 12 Months Intent-to-treat Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 27

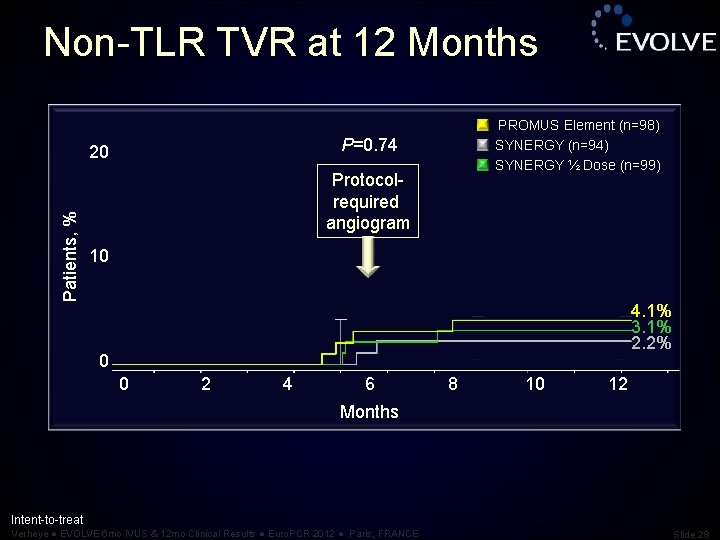
Non-TLR TVR at 12 Months P=0. 74 20 Patients, % PROMUS Element (n=98) SYNERGY (n=94) SYNERGY ½ Dose (n=99) Protocolrequired angiogram 10 4. 1% 3. 1% 2. 2% 0 0 2 4 6 8 10 12 Months Intent-to-treat Verheye ● EVOLVE 6 mo IVUS & 12 mo Clinical Results ● Euro. PCR 2012 ● Paris, FRANCE Slide 28
 Ivus ffr
Ivus ffr Nissan in hebrew
Nissan in hebrew Sidereal vs synodic moon
Sidereal vs synodic moon Ivus
Ivus Ivus
Ivus Ivus
Ivus Intravascular ultrasound
Intravascular ultrasound Parallel wire technique
Parallel wire technique Dcb clinical outcomes
Dcb clinical outcomes Nursing process steps
Nursing process steps Example of learning objectives
Example of learning objectives Collaborative interventions nursing
Collaborative interventions nursing Learning outcomes for direct and indirect speech
Learning outcomes for direct and indirect speech Monitor is input or output device
Monitor is input or output device Examples of ifsp outcomes and strategies
Examples of ifsp outcomes and strategies Learning outcomes of work and energy
Learning outcomes of work and energy Learning outcomes of profit and loss
Learning outcomes of profit and loss Learning objectives of work and energy
Learning objectives of work and energy The power of the church chapter 13 section 4
The power of the church chapter 13 section 4 Learning outcomes of ascending and descending order
Learning outcomes of ascending and descending order Aims objectives and outcomes
Aims objectives and outcomes Causes of otto invades italy on pope's behalf
Causes of otto invades italy on pope's behalf Ancient rome outcomes geography and early republic
Ancient rome outcomes geography and early republic Past perfect tense of wake up
Past perfect tense of wake up Did you see jody's new dog yesterday
Did you see jody's new dog yesterday Jessica and matt have been together for 1 month
Jessica and matt have been together for 1 month In the afylin framework learning outcomes are arranged
In the afylin framework learning outcomes are arranged Learning outcomes of water cycle
Learning outcomes of water cycle Lesson plan on notice writing
Lesson plan on notice writing