Silicones Phosphazenes Dr MAHMOUD NAJIM 2020 Introduction Inorganic






- Slides: 16

Silicones & Phosphazenes Dr MAHMOUD NAJIM 2020

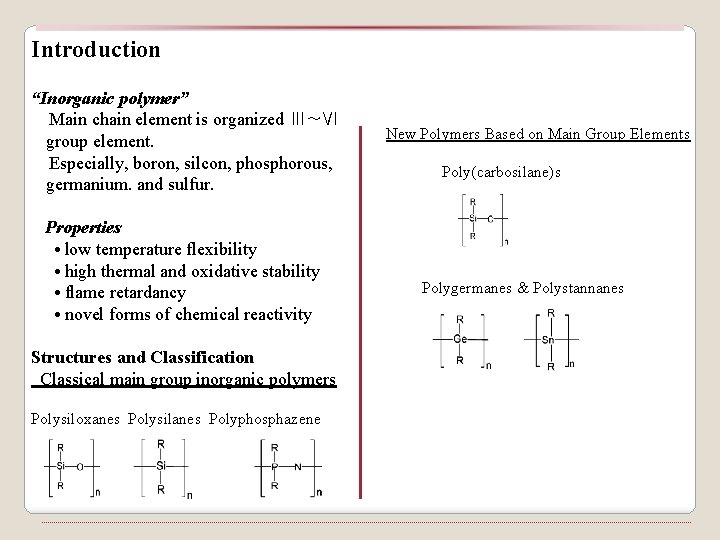
Introduction “Inorganic polymer” Main chain element is organized Ⅲ~Ⅵ group element. Especially, boron, silcon, phosphorous, germanium. and sulfur. Properties • low temperature flexibility • high thermal and oxidative stability • flame retardancy • novel forms of chemical reactivity Structures and Classification Classical main group inorganic polymers Polysiloxanes Polysilanes Polyphosphazene New Polymers Based on Main Group Elements Poly(carbosilane)s Polygermanes & Polystannanes

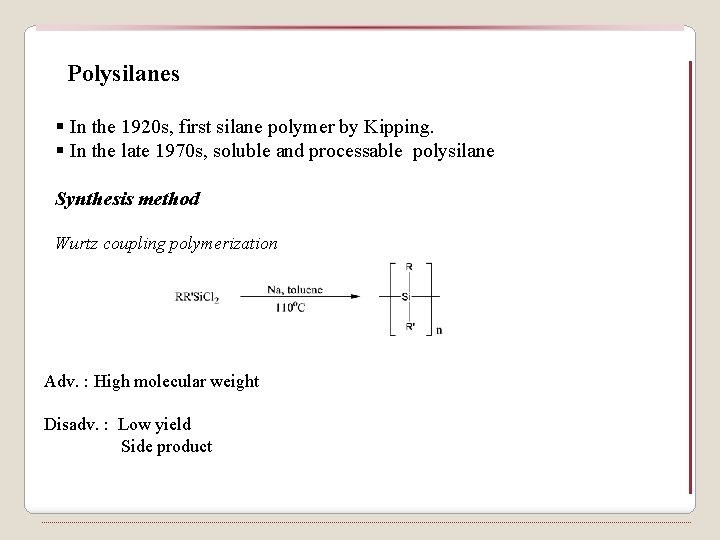
Polysilanes § In the 1920 s, first silane polymer by Kipping. § In the late 1970 s, soluble and processable polysilane Synthesis method Wurtz coupling polymerization Adv. : High molecular weight Disadv. : Low yield Side product

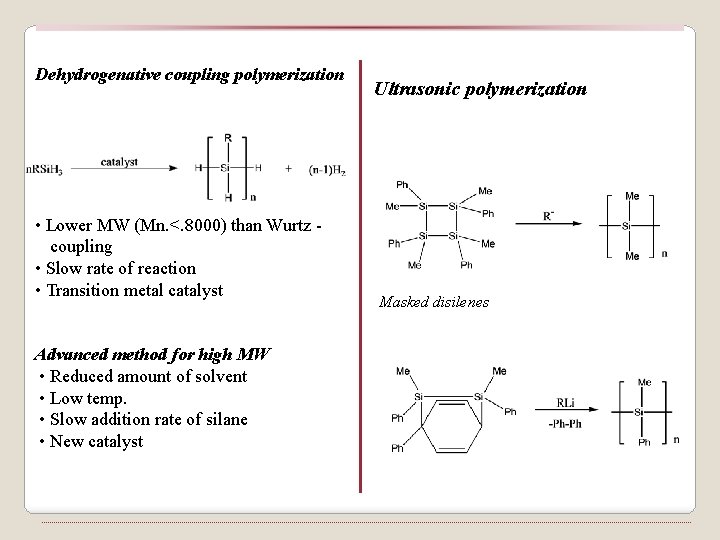
Dehydrogenative coupling polymerization • Lower MW (Mn. <. 8000) than Wurtz coupling • Slow rate of reaction • Transition metal catalyst Advanced method for high MW • Reduced amount of solvent • Low temp. • Slow addition rate of silane • New catalyst Ultrasonic polymerization Masked disilenes

Electrochemical polymerization: • Unlike the wurtz methods, • Very low temperature • Potential required for reductive formation(-1. 3 to -2. 1 V) • Polysilane forms initially coating on the cathode

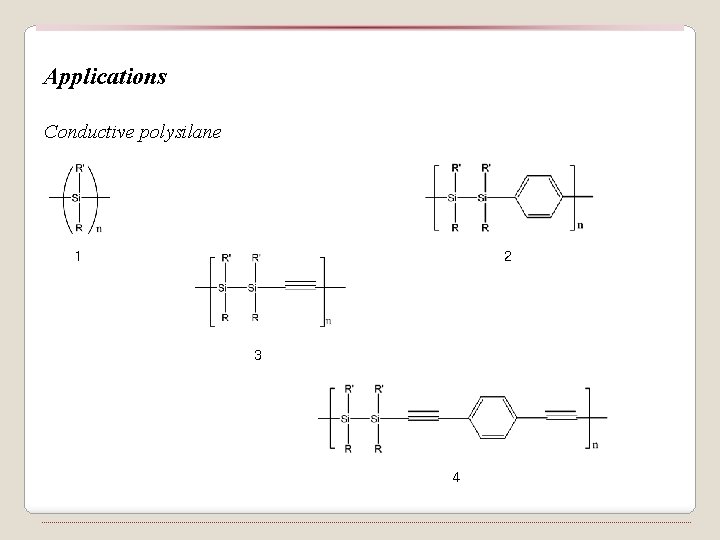
Applications Conductive polysilane 1 2 3 4

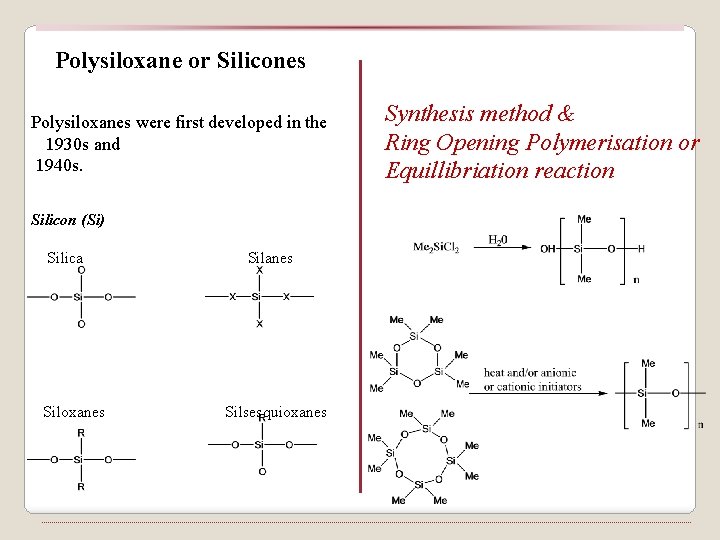
Polysiloxane or Silicones Polysiloxanes were first developed in the 1930 s and 1940 s. Silicon (Si) Silica Siloxanes Silsesquioxanes Synthesis method & Ring Opening Polymerisation or Equillibriation reaction

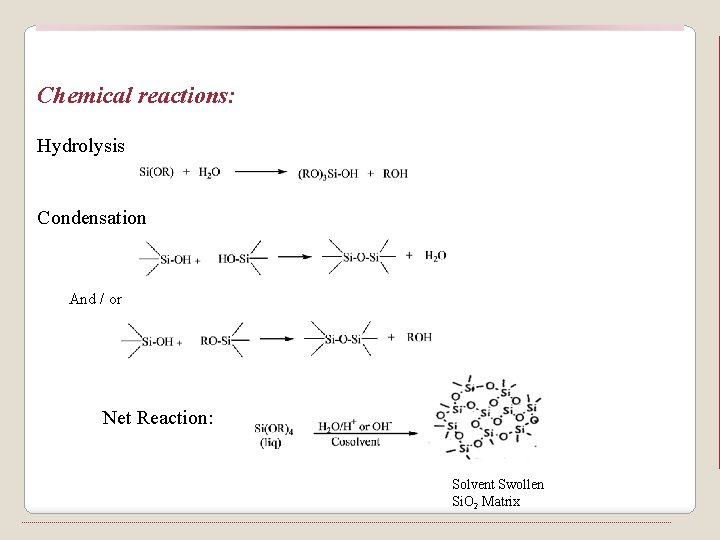
Chemical reactions: Hydrolysis Condensation And / or Net Reaction: Solvent Swollen Si. O 2 Matrix

Properties: • • Highly thermally and chemically stable. Water repellent. Used for low temp. lubrication. LMW silicon polymers are soluble in organic solvents. Good insulators. Resistant to oxidation. Have non-sticking and anti-foaming properties. Applications: • • • Used as greases, varnishes and lubricants. Used for water proofing in electrical condensers. Also used for various purposes at low temperatures. Used in medicinal and cosmetic implants because of low toxicity. Used for high temp. oil baths, high vaccum pumps, etc.

Silicone fluids & Silicone elastomers Silicone fluids are linear polysiloxanes of 50 -200 units. Prepared by treating a mixture of tetrakis cyclodimethyl siloxanes and hexamethyl disiloxane with a small quantity of 100% sulphuric acid. These are used as water repellents, insulating material, hydraulic oils and various lubrication purposes. Used in processing of cooking oils and fruit juices due to its non-toxicity. Silicone elastomers are long chain polymers made of 6000 -600000 silicone units. Have high molecular weights. These can be vulcanised to give rubber. Vulcanisation is the formation of cross-linked chains. Silicone resins are solvent solutions of branched chain siloxanes containing residual hydroxyl groups.

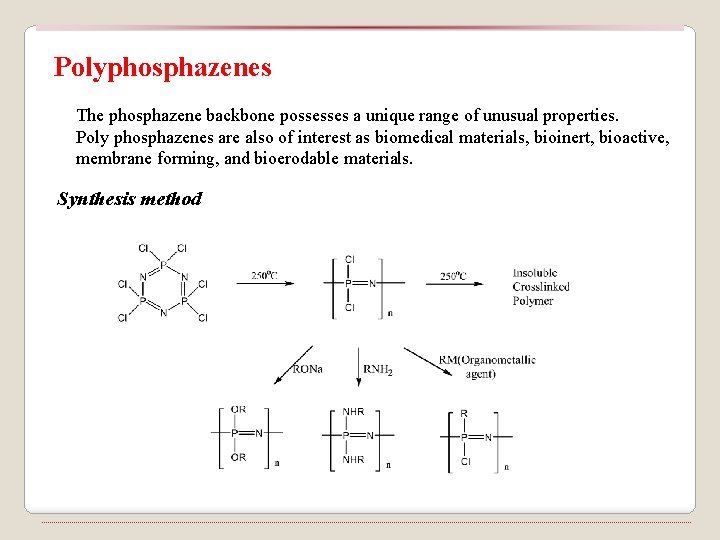
Polyphosphazenes The phosphazene backbone possesses a unique range of unusual properties. Poly phosphazenes are also of interest as biomedical materials, bioinert, bioactive, membrane forming, and bioerodable materials. Synthesis method

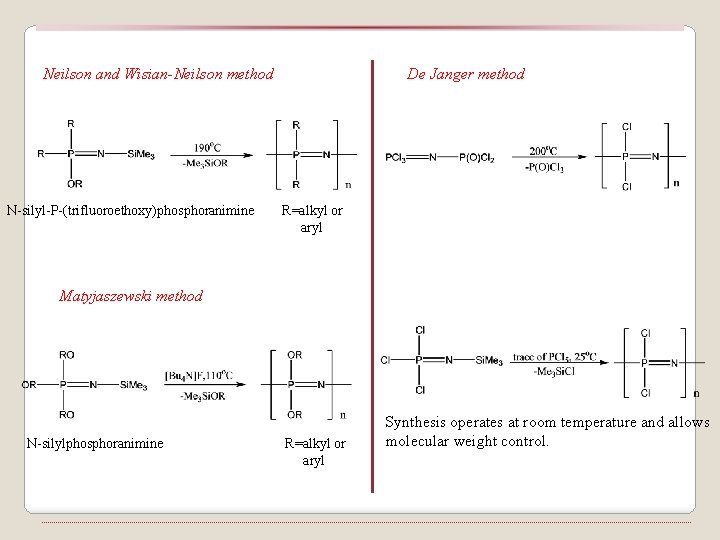
Neilson and Wisian-Neilson method N-silyl-P-(trifluoroethoxy)phosphoranimine De Janger method R=alkyl or aryl Matyjaszewski method N-silylphosphoranimine R=alkyl or aryl Synthesis operates at room temperature and allows molecular weight control.

Applications: • Halides of phosphazenes are used as rigid plastics, expanded foam and fibres. • Used as water and fire proof materials. • These are unaffected by oil, petrol and other solvents. • Used as catalysts in manufacturing of silicones. • Used as flexible plastics.

Structure and bonding

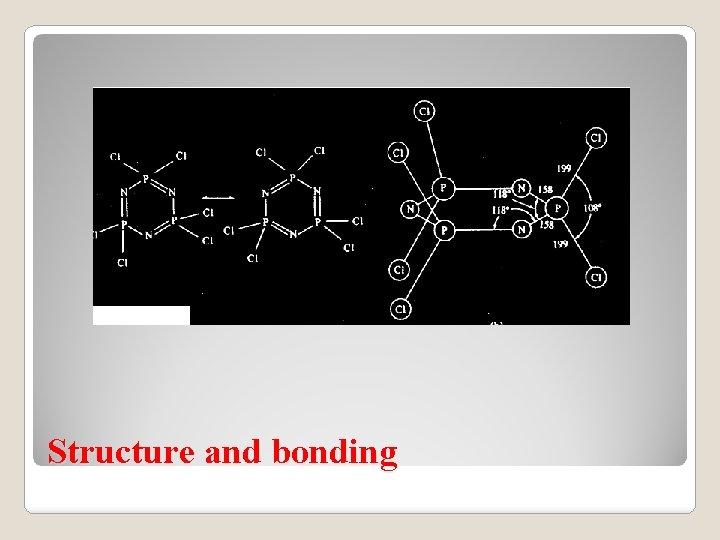
The halide trimers consist of planar six membered rings. The bond angles are consistent with SP 2 hybridization of the nitrogen and approximately SP 3 hybridization of the phosphorous. Two of the SP 2 orbital of nitrogen, containing one electron each, are used for 's' bonding and the third contains a lone pair of electron. This leaves one electron for the unhybridised PZ orbital. The four SP 3 hybrid orbital (containing four electrons) of phosphorous are used for 's' bonding leaving a fifth electron to occupy a 'd' orbital. All phosphazenes are not planar. The dxz orbital of the phosphorous atom overlaps with the pz orbital of the nitrogen atom adjacent to it. The dyz orbital which is perpendicular to the dxz, can also overlap with the p z orbital of Nitrogen. There may be in–plane – P bonding between the sp 2 non bonding orbital of nitrogen and the dxy and for dx 2 – y 2 orbital of the phosphorous. Structure studies

Nature of bonding (Orbital form)
 Silicones and phosphazenes notes
Silicones and phosphazenes notes Uses of silicone
Uses of silicone Silicone and phosphazenes
Silicone and phosphazenes Silicones and phosphazenes notes
Silicones and phosphazenes notes Introduction to pharmaceutical inorganic chemistry
Introduction to pharmaceutical inorganic chemistry Introduction to inorganic chemistry
Introduction to inorganic chemistry Mahmoud abdelfattah age
Mahmoud abdelfattah age Passport mahmoud darwish
Passport mahmoud darwish Salman mustafa
Salman mustafa Mahmoud arafa
Mahmoud arafa Boudarene mahmoud
Boudarene mahmoud Mahmoud sarmini md
Mahmoud sarmini md Adp antagonist drugs
Adp antagonist drugs Mahmoud khattab md
Mahmoud khattab md Hana mahmoud
Hana mahmoud Wacker silicones
Wacker silicones Sempure 60
Sempure 60


























