SEWAGE CHARACTERISTICS SEWAGE CHARACTERISTICS Composition 99 0 Water




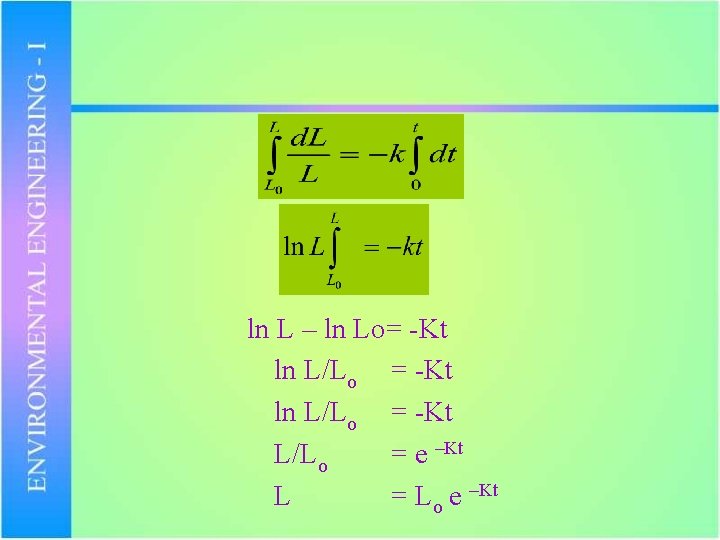









- Slides: 30

SEWAGE CHARACTERISTICS

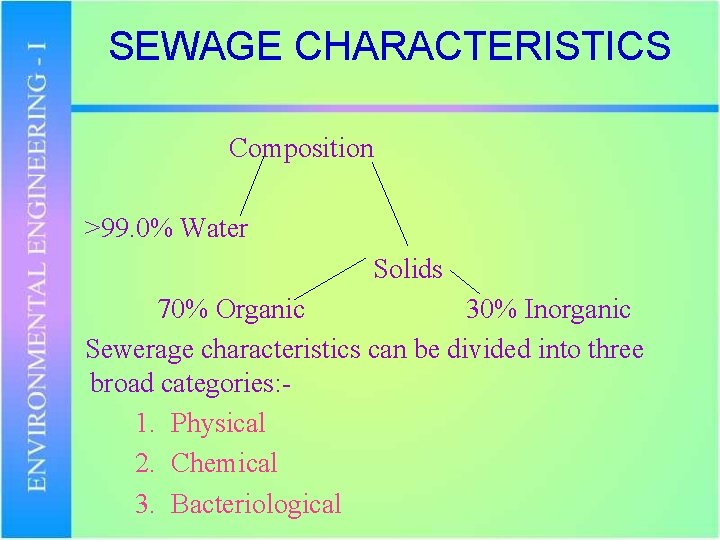
SEWAGE CHARACTERISTICS Composition >99. 0% Water Solids 70% Organic 30% Inorganic Sewerage characteristics can be divided into three broad categories: 1. Physical 2. Chemical 3. Bacteriological

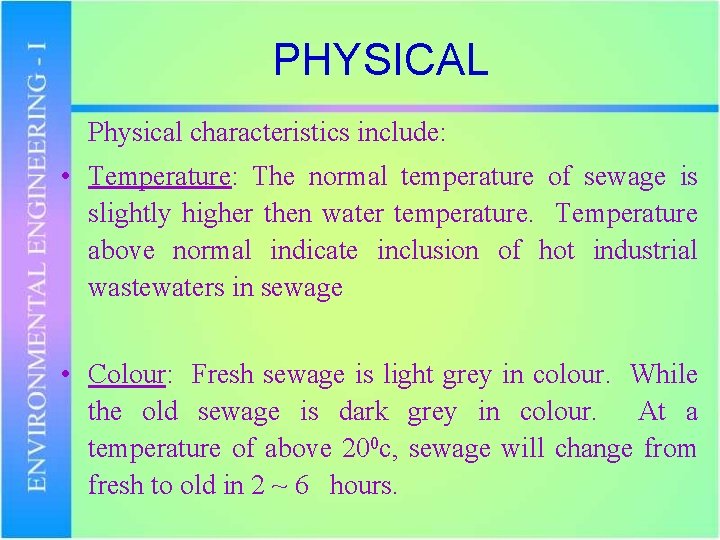
PHYSICAL Physical characteristics include: • Temperature: The normal temperature of sewage is slightly higher then water temperature. Temperature above normal indicate inclusion of hot industrial wastewaters in sewage • Colour: Fresh sewage is light grey in colour. While the old sewage is dark grey in colour. At a temperature of above 200 c, sewage will change from fresh to old in 2 ~ 6 hours.

• Odour: Fresh domestic sewage has a slightly soapy or oil odour. Stale sewage has a pronounced odour of Hydrogen Sulphide (H 2 S). • Solids: - Solids in sewage may be suspended or in solution solids are a measure of the strength of sewage.

CHEMICAL Sewage contain both organic and inorganic chemicals. All the test representing these organic and inorganic constituents come under the heading of chemical characteristics. Test like BOD, COD, NITORGON, PHOSPHOURS, ALKALINITY etc give the chemical characteristics of sewage.

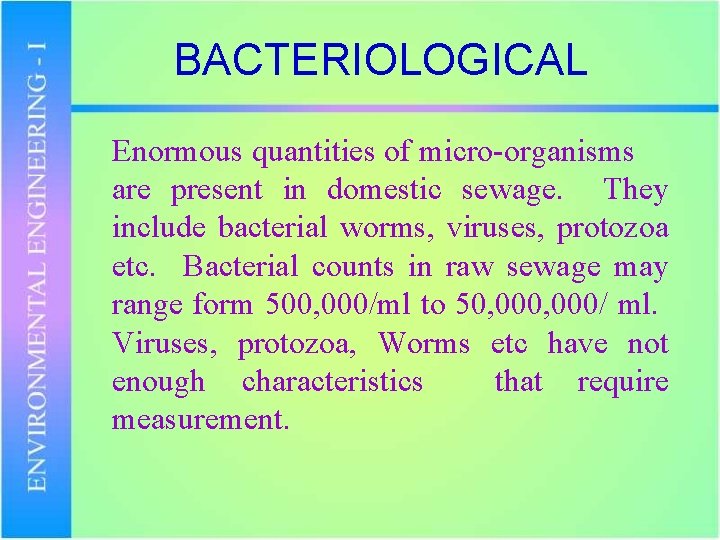
BACTERIOLOGICAL Enormous quantities of micro-organisms are present in domestic sewage. They include bacterial worms, viruses, protozoa etc. Bacterial counts in raw sewage may range form 500, 000/ml to 50, 000/ ml. Viruses, protozoa, Worms etc have not enough characteristics that require measurement.

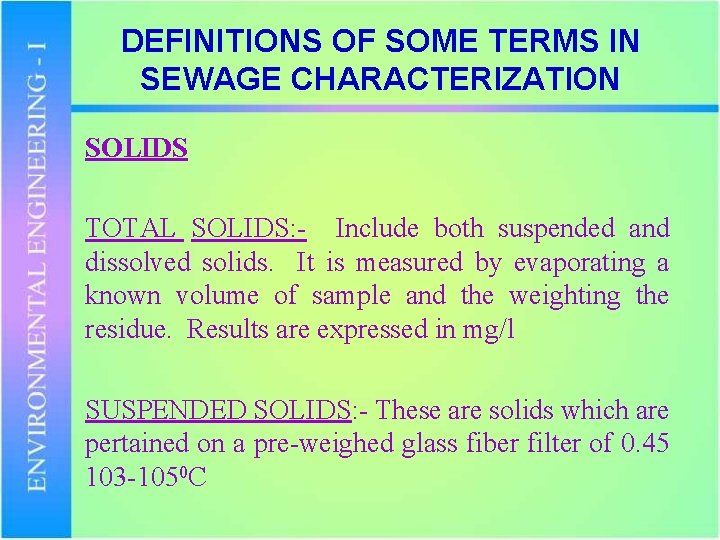
DEFINITIONS OF SOME TERMS IN SEWAGE CHARACTERIZATION SOLIDS TOTAL SOLIDS: - Include both suspended and dissolved solids. It is measured by evaporating a known volume of sample and the weighting the residue. Results are expressed in mg/l SUSPENDED SOLIDS: - These are solids which are pertained on a pre-weighed glass fiber filter of 0. 45 103 -1050 C

DISSOLVED SOLID: - Filtrate which has passed thought 0. 45µ filter is evaporated in chine dish. The residue gives the dissolved solids. SETTLEABLE SOLIDS: - It is the fraction of the solids that will settle in an imhoff cone in 30 -60 minutes. These are expressed as mg/l.

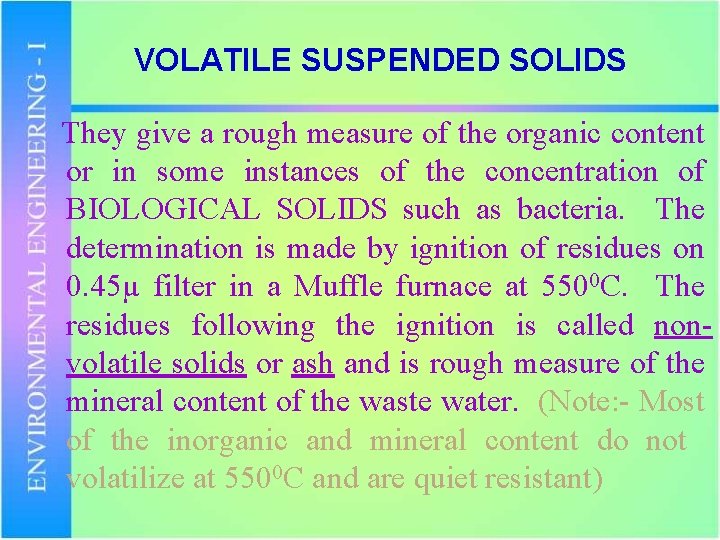
VOLATILE SUSPENDED SOLIDS They give a rough measure of the organic content or in some instances of the concentration of BIOLOGICAL SOLIDS such as bacteria. The determination is made by ignition of residues on 0. 45µ filter in a Muffle furnace at 5500 C. The residues following the ignition is called nonvolatile solids or ash and is rough measure of the mineral content of the waste water. (Note: - Most of the inorganic and mineral content do not volatilize at 5500 C and are quiet resistant)

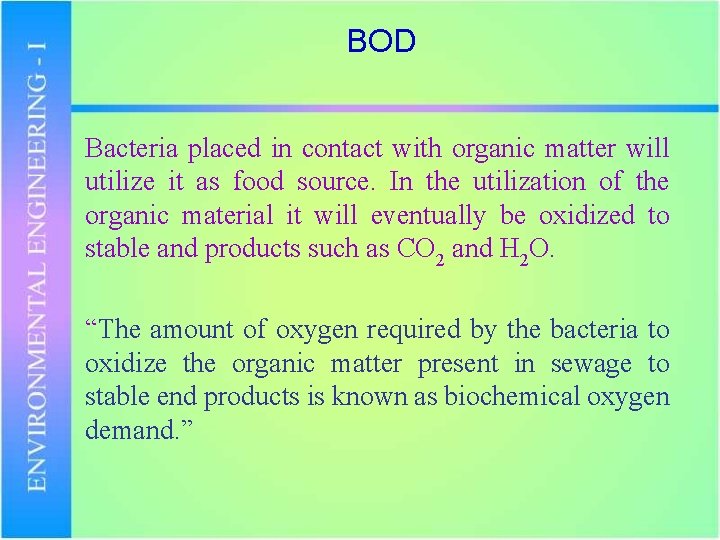
BOD Bacteria placed in contact with organic matter will utilize it as food source. In the utilization of the organic material it will eventually be oxidized to stable and products such as CO 2 and H 2 O. “The amount of oxygen required by the bacteria to oxidize the organic matter present in sewage to stable end products is known as biochemical oxygen demand. ”

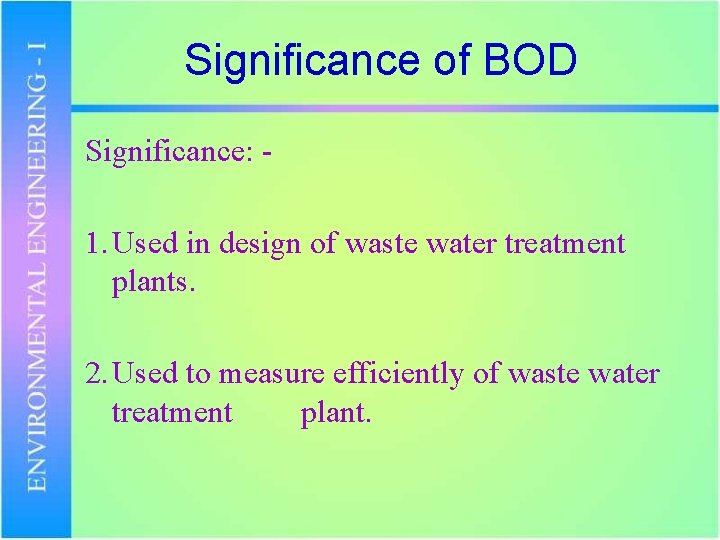
Significance of BOD Significance: - 1. Used in design of waste water treatment plants. 2. Used to measure efficiently of waste water treatment plant.

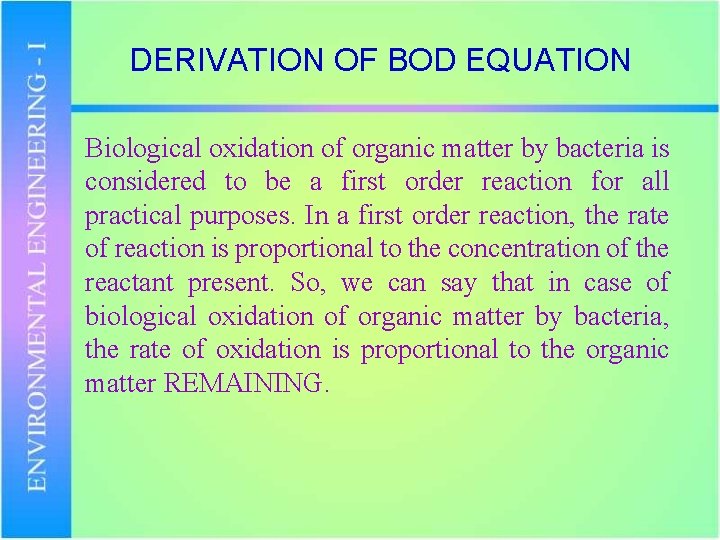
DERIVATION OF BOD EQUATION Biological oxidation of organic matter by bacteria is considered to be a first order reaction for all practical purposes. In a first order reaction, the rate of reaction is proportional to the concentration of the reactant present. So, we can say that in case of biological oxidation of organic matter by bacteria, the rate of oxidation is proportional to the organic matter REMAINING.

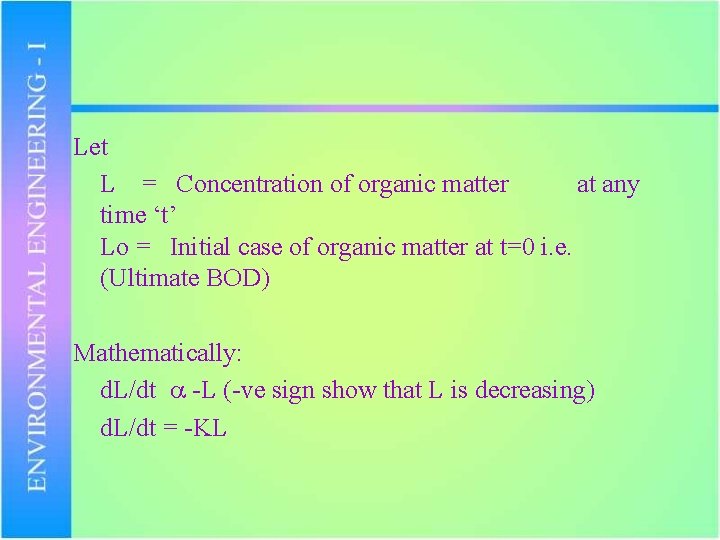
Let L = Concentration of organic matter at any time ‘t’ Lo = Initial case of organic matter at t=0 i. e. (Ultimate BOD) Mathematically: d. L/dt -L (-ve sign show that L is decreasing) d. L/dt = -KL

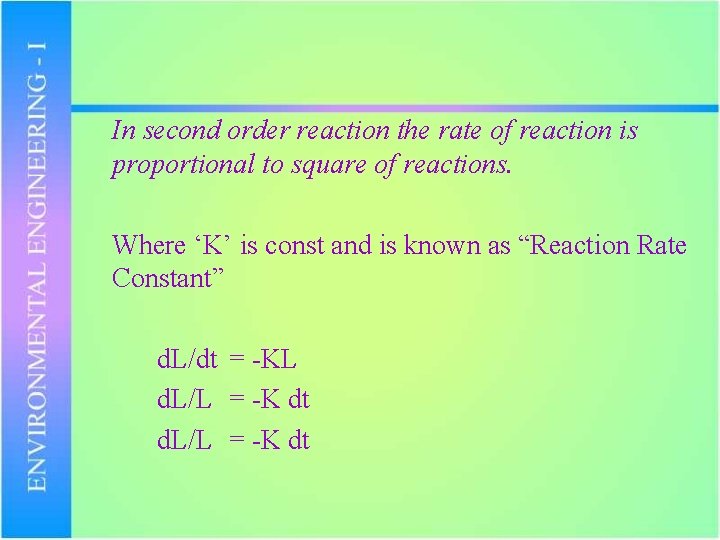
In second order reaction the rate of reaction is proportional to square of reactions. Where ‘K’ is const and is known as “Reaction Rate Constant” d. L/dt = -KL d. L/L = -K dt
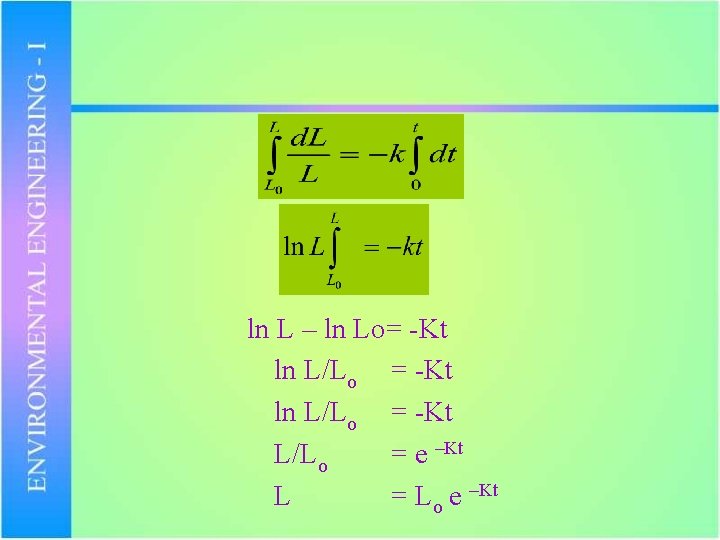
ln L – ln Lo= -Kt ln L/Lo = -Kt L/Lo = e –Kt L = Lo e –Kt

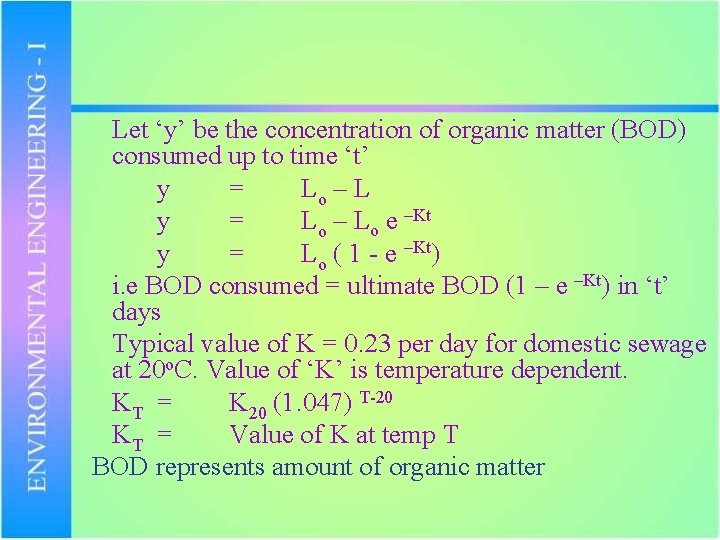
Let ‘y’ be the concentration of organic matter (BOD) consumed up to time ‘t’ y = Lo – Lo e –Kt y = Lo ( 1 - e –Kt) i. e BOD consumed = ultimate BOD (1 – e –Kt) in ‘t’ days Typical value of K = 0. 23 per day for domestic sewage at 20 o. C. Value of ‘K’ is temperature dependent. KT = K 20 (1. 047) T-20 KT = Value of K at temp T BOD represents amount of organic matter

L BOD mg/l Lo y ‘t’ time

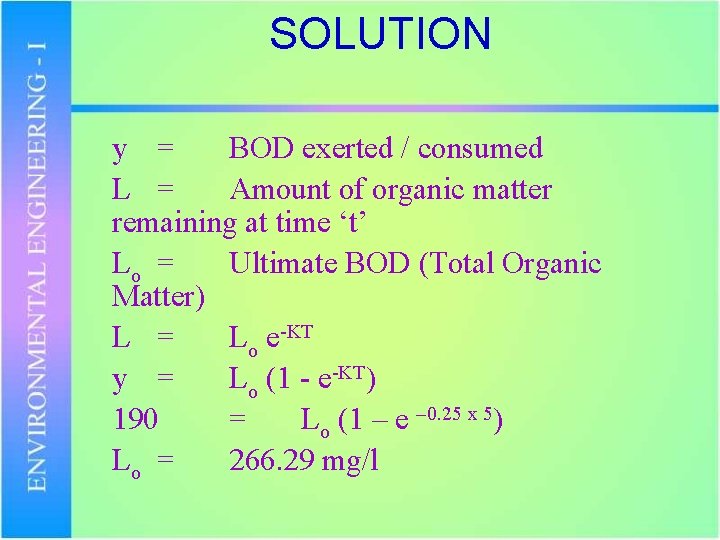
Problem The 5 day BOD of waste water is 190 mg/l. determine ultimate BOD assuming K = 0. 25 per day

SOLUTION y = BOD exerted / consumed L = Amount of organic matter remaining at time ‘t’ Lo = Ultimate BOD (Total Organic Matter) L = Lo e-KT y = Lo (1 - e-KT) 190 = Lo (1 – e – 0. 25 x 5) Lo = 266. 29 mg/l

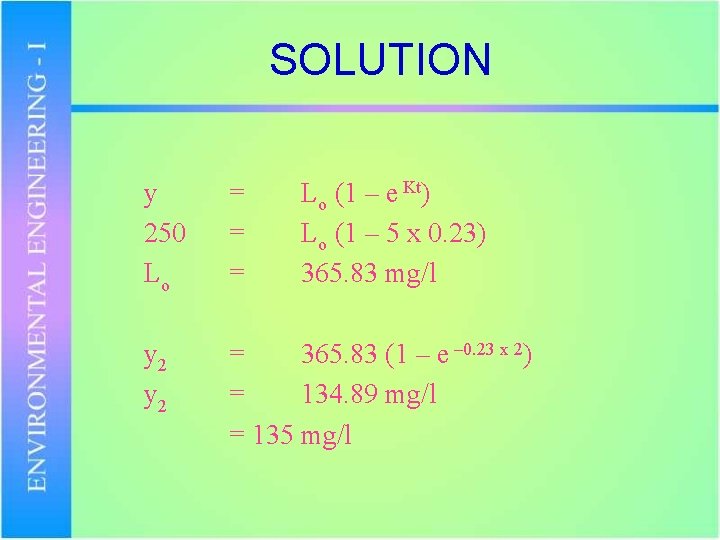
Problem Calculate the ultimate BOD for a sewage whose 5 day BOD at 20 o. C is 250 mg/l. Assume K = 0. 23 per day what will be BOD after 2 days.

SOLUTION y 250 Lo y 2 = = = Lo (1 – e Kt) Lo (1 – 5 x 0. 23) 365. 83 mg/l = 365. 83 (1 – e – 0. 23 x 2) = 134. 89 mg/l = 135 mg/l

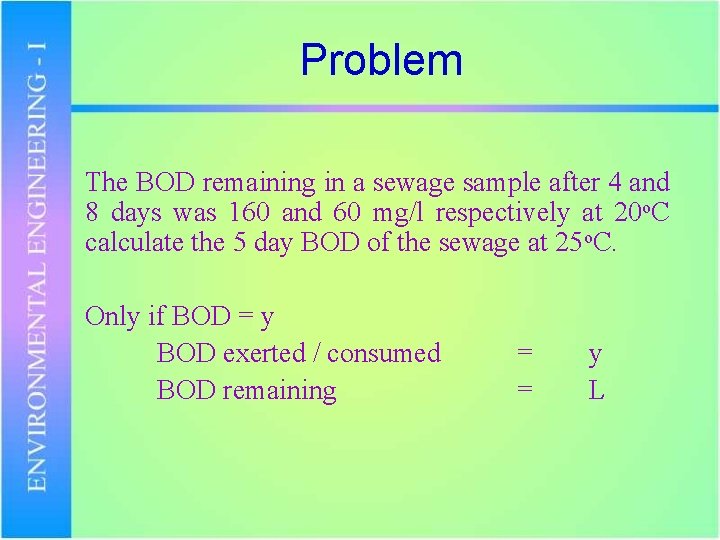
Problem The BOD remaining in a sewage sample after 4 and 8 days was 160 and 60 mg/l respectively at 20 o. C calculate the 5 day BOD of the sewage at 25 o. C. Only if BOD = y BOD exerted / consumed BOD remaining = = y L

SOLUTION L = Lo e-Kt 160 = Lo e-K x 4 60 = Lo e-K x 8 Lo = 160 / e – 4 K 60 = [160 / e – 4 K ] e–K 8 60 = 160 e – 8 K +4 K 0. 375 = e – 4 K ln (0. 375) = -4 k K = 0. 245 per day

Lo At 25 o. C K 25 = Y 5 = = 160/e – 4 x 0. 245 K 20 (1. 047) T-20 0. 245 (1. 047) 25 -20 0. 308 per day Lo (1 - e –Kt) 426. 3 (1 – e – 0. 308 x 5) 335 mg/l =426. 3 mg/l

CHEMICAL OXYGEN DEMAND It is the amount of oxygen required to oxidize organic matter chemically (biodegradable and nonbiodegradable) by using a strong chemical oxidizing agent. (K 2 Cr 2 O 7) in an acidic medium. For a single waste water sample the value of COD will always be greater then BOD. The oxidant (K 2 Cr 2 O 7) remaining is found out to find K 2 Cr 2 O 7 considered COD and BOD can be interrelated.

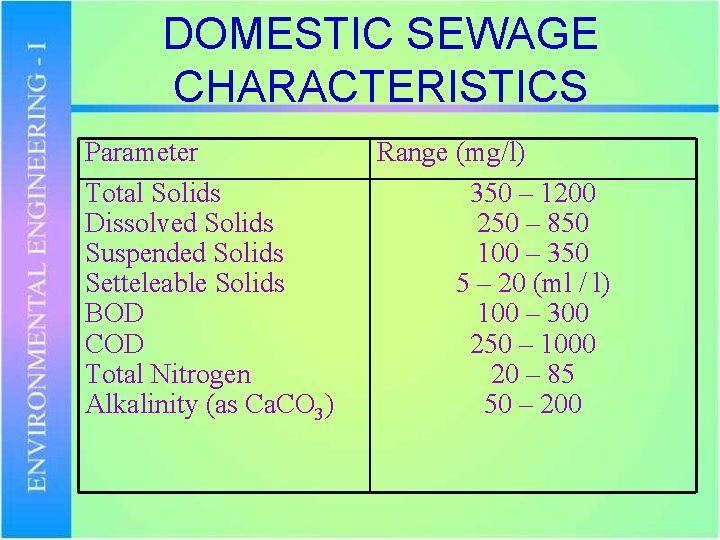
DOMESTIC SEWAGE CHARACTERISTICS Parameter Total Solids Dissolved Solids Suspended Solids Setteleable Solids BOD COD Total Nitrogen Alkalinity (as Ca. CO 3) Range (mg/l) 350 – 1200 250 – 850 100 – 350 5 – 20 (ml / l) 100 – 300 250 – 1000 20 – 85 50 – 200

Water Resources Management

Definitions • Hydrology – the scientific study of the properties, distribution and effects of water on the earth’s surface, in the soil and underlying rocks and in the atmosphere • Water resources management -- control and utilization of water for beneficial uses or to avoid adverse impacts – drinking water supply – irrigation – industrial water supply -- flooding -- drought -- subsidence

Determining Water Demand • Average daily water consumption • Ability to meet continuing demand over critical periods • Estimate stored water requirement quantities • Peak demand rates (~ 2. 2 x daily average flow for metered dwellings) • Size of plumbing and piping, pressure losses • Estimate storage requirements during periods of peak water demand

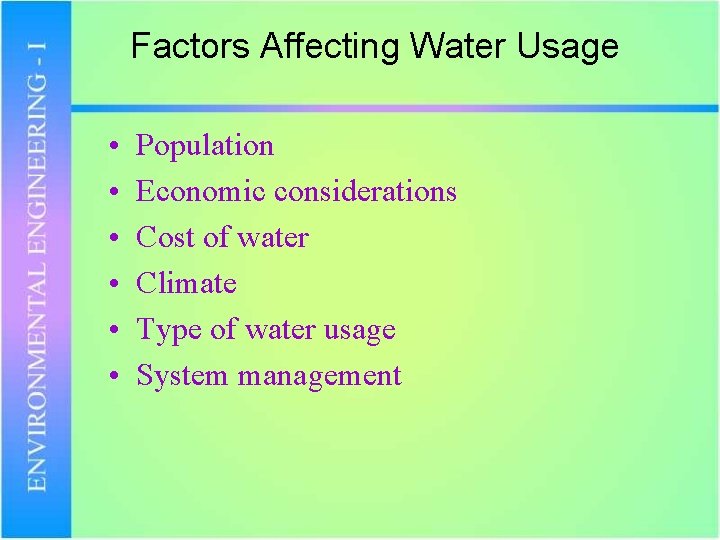
Factors Affecting Water Usage • • • Population Economic considerations Cost of water Climate Type of water usage System management
 Water and water and water water
Water and water and water water Fannings formula
Fannings formula Ce certification sewage drainage water pump
Ce certification sewage drainage water pump Water treated from sewage treatment plant
Water treated from sewage treatment plant Sewage water
Sewage water Characteristics of wastewater
Characteristics of wastewater Grit chamber design example
Grit chamber design example John martinko
John martinko Sewage cleanup hanahan
Sewage cleanup hanahan Conservancy and water carriage system
Conservancy and water carriage system Bs en 12566
Bs en 12566 Sewage pump sizing
Sewage pump sizing Sewage sickness
Sewage sickness Where can we find rotating arm sprays in sewage treatment?
Where can we find rotating arm sprays in sewage treatment? Local guide program
Local guide program Conclusion of wastewater treatment
Conclusion of wastewater treatment Percent composition
Percent composition How to find empirical formula from percentages
How to find empirical formula from percentages School water audit
School water audit Fresh water allowance
Fresh water allowance Water o water
Water o water Estimating products of fractions
Estimating products of fractions A paved blacktop parking lot was built
A paved blacktop parking lot was built Water exchanger
Water exchanger Fresh water meets salt water
Fresh water meets salt water Warm water rises in a lake. cold water descends.
Warm water rises in a lake. cold water descends. Water water everywhere project
Water water everywhere project Sources of water sources of water
Sources of water sources of water Unit 11 water water everywhere
Unit 11 water water everywhere Water soluble vitamins characteristics
Water soluble vitamins characteristics Sanitary well psm
Sanitary well psm