SEWAGE PUMPING STATIONS SEWAGE PUMPING STATIONS Purpose These

















- Slides: 35

SEWAGE PUMPING STATIONS

SEWAGE PUMPING STATIONS Purpose: These are required to ELEVATE and TRANSPORT wastewater when – Continuation of gravity flow is no longer feasible and there is a need to raise the HGL of sewer. – Any obstacle lies in the path of sewer (e. g. river, canal etc) – Receiving stream is higher than the sewer.

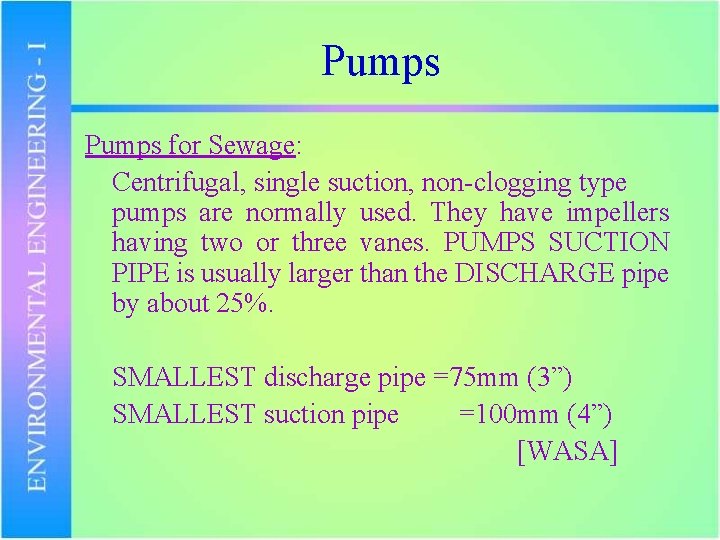
Pumps for Sewage: Centrifugal, single suction, non-clogging type pumps are normally used. They have impellers having two or three vanes. PUMPS SUCTION PIPE is usually larger than the DISCHARGE pipe by about 25%. SMALLEST discharge pipe =75 mm (3”) SMALLEST suction pipe =100 mm (4”) [WASA]

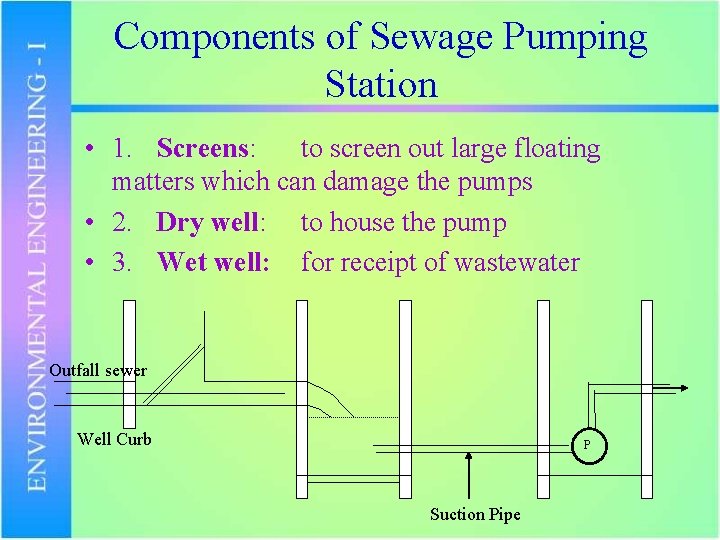
Components of Sewage Pumping Station • 1. Screens: to screen out large floating matters which can damage the pumps • 2. Dry well: to house the pump • 3. Wet well: for receipt of wastewater Outfall sewer Well Curb P Suction Pipe

GENERAL DESIGN CONSIDERATIONS 1. More than one pump should be provided to cope with variable discharge. Two pumps for small P. S and more than two for large P. S should be used: a. Minimum flow b. Average flow c. Maximum flow 2. Total pumping capacity of the P. S must be equal to the peak sewage flow.

GENERAL DESIGN CONSIDERATIONS 3. Standby pump MUST be provided at the P. S. Its capacity should be at least 50% of peak sewage flow. 4. Alternate source of power MUST be provided at P. S. (Either power from two feeders or a diesel operated pump be provided) 5. Pumps should be SELF PRIMING TYPE and should operate under +ve suction head.

GENERAL DESIGN CONSIDERATIONS 6. Each pump should have an individual intake. 7. Screens with 50 mm opening be provided at pump suction to avoid entrance of big particles in pumps. 8. Size of dry well should be sufficient to house pumping machinery.

GENERAL DESIGN CONSIDERATIONS 7. Dry well be provided with pumps which are usually RECIPROCATING PUMPS to pump out sewage leaks in dry well. 8. Sluice valves must be provided at suction and non-return valve at the delivery side. 9. Detention time in wet well should not be greater than 30 minutes to avoid septic conditions.

Design of Pumping station Design refer to finding the OPERATING VOLUME of wet well. A wet well has to fulfil two requirements: 1. Pumps should not be started and stopped frequently to avoid overheating of motors. Time between two successive start ups of the pumps should be MORE THAN MINIMUM manufacturer. Cycle time =5 ~ 10 minutes for small pumps 15 ~ 20 minutes for large pumps Cycle time can be defined as (time between two successive start ups of the pumps)

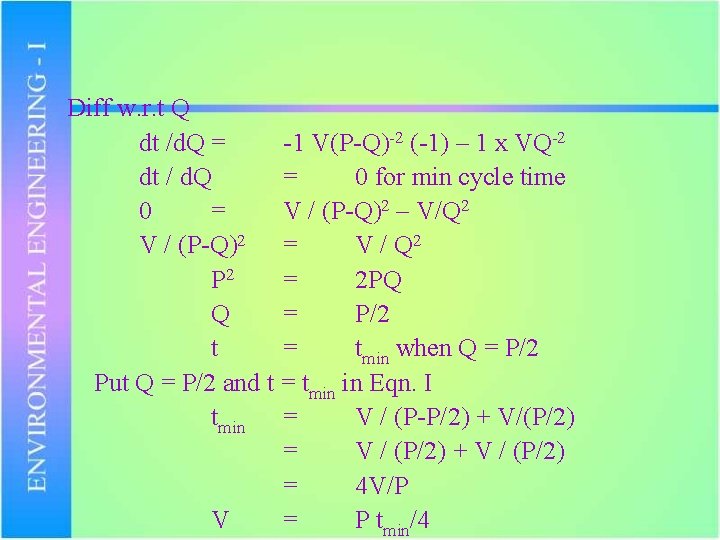
2. Detention time in wet well at average flow should not be more than 30 minutes to avoid SEPTIC CONDITIONS. Cycle time t t t = = Time on + Time off Time to empty + Time to fill V/(P-Q) + V/Q … I V(P-Q)-1 + VQ-1

Diff w. r. t Q dt /d. Q = -1 V(P-Q)-2 (-1) – 1 x VQ-2 dt / d. Q = 0 for min cycle time 0 = V / (P-Q)2 – V/Q 2 V / (P-Q)2 = V / Q 2 P 2 = 2 PQ Q = P/2 t = tmin when Q = P/2 Put Q = P/2 and t = tmin in Eqn. I tmin = V / (P-P/2) + V/(P/2) = V / (P/2) + V / (P/2) = 4 V/P V = P tmin/4

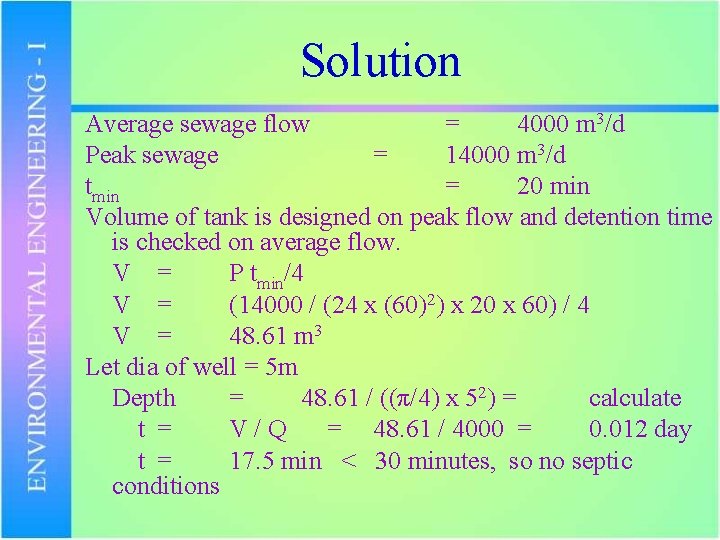
PROBLEM Design a wet well if the average sewage flow is 4000 m 3/d and peak sewage flow is 14000 m 3/d. The motor pump has a minimum cycle time of 20 min. Also check the detention time to avoid septic condition.

Solution Average sewage flow = 4000 m 3/d Peak sewage = 14000 m 3/d tmin = 20 min Volume of tank is designed on peak flow and detention time is checked on average flow. V = P tmin/4 V = (14000 / (24 x (60)2) x 20 x 60) / 4 V = 48. 61 m 3 Let dia of well = 5 m Depth = 48. 61 / ((π/4) x 52) = calculate t = V/Q = 48. 61 / 4000 = 0. 012 day t = 17. 5 min < 30 minutes, so no septic conditions

Lake Ecosystems

Definitions Limnology – the study of the ecology of inland waters. Derived from the Greek root limne meaning pool or marsh. Focused on freshwater biotic communities and the relationships of the biota to the physical, chemical and biological characteristics of the aquatic system which they are a part of.

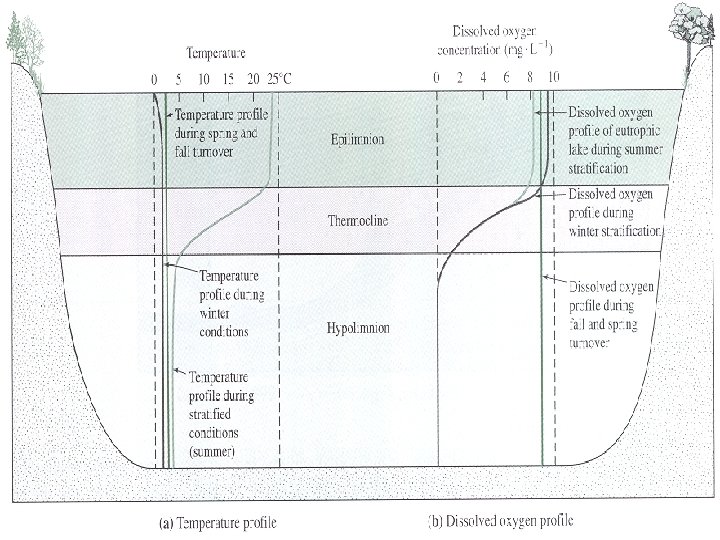
Temperature and Oxygen

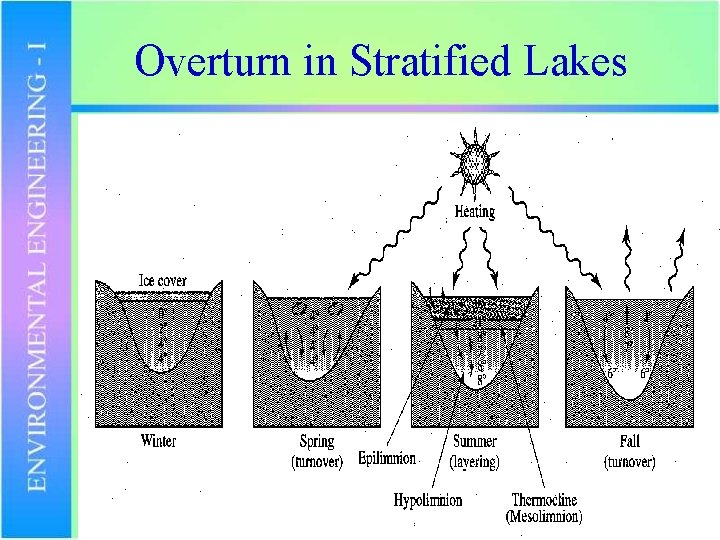
Overturn in Stratified Lakes

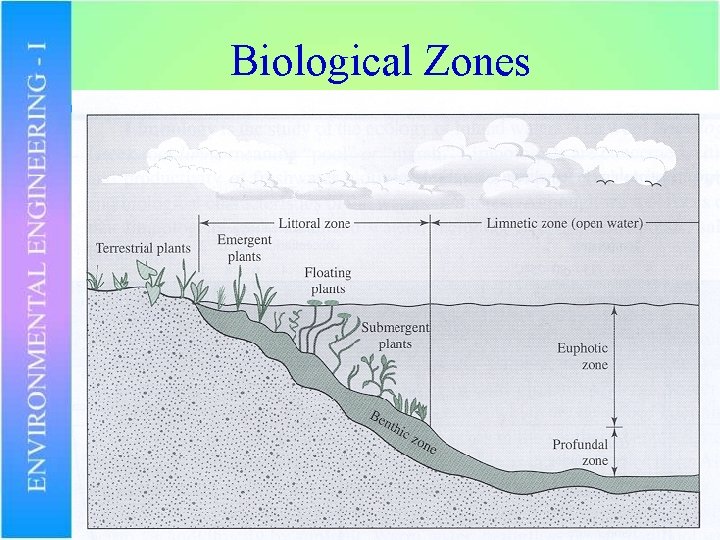
Biological Zones

Productivity • Stoichiometry of photosysnthesis (C, N, P, O & H) 106 CO 2 + 16 NO 3 - + HPO 42 - + 122 H 2 O → C 106 H 263 O 110 N 16 P + 138 O 2 • Liebig’s law of the minimum – growth will be limited by the availability of the nutrient that is least available relative to the need

Limiting Nutrients • Most freshwater systems are phosphorus limited • Inputs of the limiting nutrient will result in a productivity increase – growth of algae

Limiting Nutrients • Most marine systems are nitrogen limited • Excessive inputs will cause an algal bloom • Estimated that total phosphorus concentration in lake water should be below 0. 010 -0. 015 mg/L to prevent algal blooms

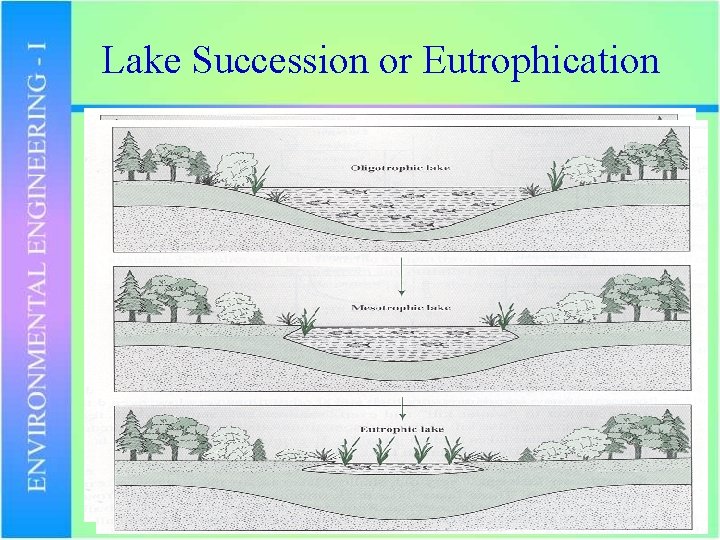
Lake Succession or Eutrophication

Perspective on Eutrophication • Eutrophication is a natural process • Some lakes have been eutrophic long before human activities could have had any effect • Aging process is thought to occur over thousands of years • Cultural eutrophication is accelerated aging due to human influences • May occur over tens of years

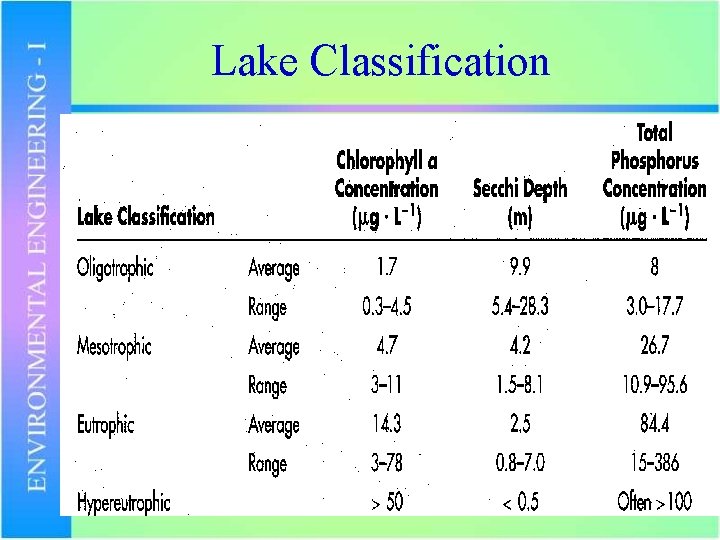
Lake Classification

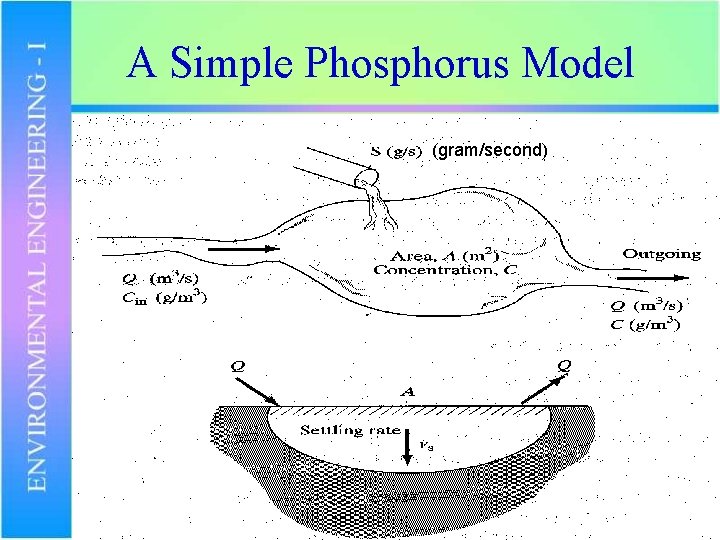
A Simple Phosphorus Model (gram/second)

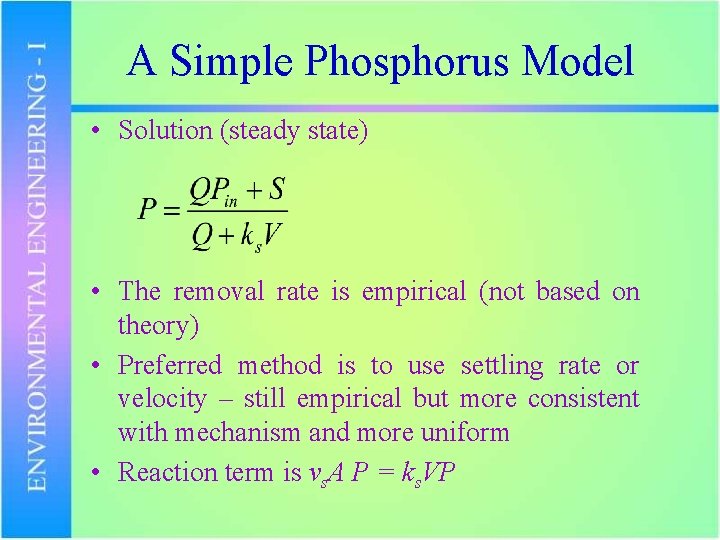
A Simple Phosphorus Model S = loading from point source (g/s) P = P concentration in lake (g/m 3) Pin= P concentration in incoming stream (g/m 3) Q = stream inflow and outflow (m 3/s) ks = P removal rate (L/s) A = surface area of the lake (m 2) V = volume of the lake (m 3)

A Simple Phosphorus Model • Solution (steady state) • The removal rate is empirical (not based on theory) • Preferred method is to use settling rate or velocity – still empirical but more consistent with mechanism and more uniform • Reaction term is vs. A P = ks. VP

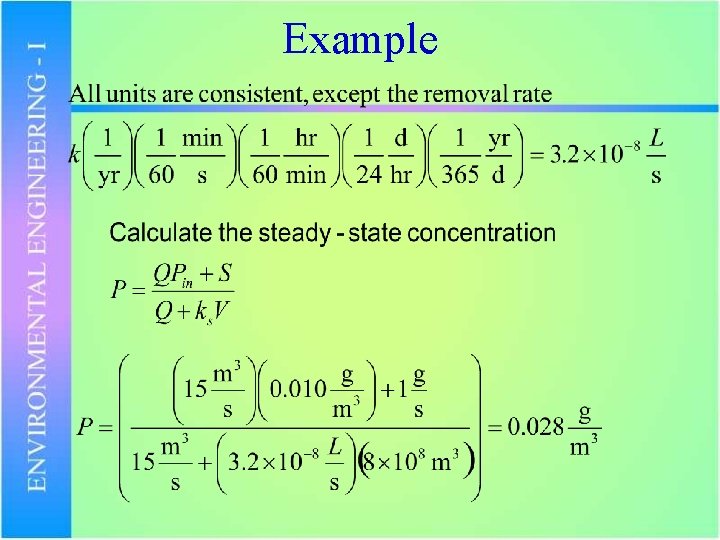
Example • A phosphorus limited lake with a volume of 8 x 108 m 3 is fed by a 15 m 3/s stream with a P concentration of 0. 010 mg/L. – Estimate the total steady-state P concentration in the lake – How would you classify the lake? – What level of P removal would be required in the point source to keep the P level below 0. 015 mg/L?

Example

Acidification of Lakes • Rainfall naturally has a p. H of 5. 6 due to CO 2 dissolution • Acid rain is defined as p. H<5. 6 • Acid deposition can be wet (rain, snow, fog) or dry (particles, gases, aerosols) • S in coal: SO 2 + H 2 O → H 2 SO 4 (sulfuric acid) • NO 2 from combustion, primarily vehicles: NO 2 + H 2 O → HNO 3 (nitric acid)

Effects of Acid Rain • Degrades building materials especially limestone, marble, metals, paints • p. H reduction in surface waters – many species can not tolerate p. H < 5 • solubilizes metals leading to increased toxicity

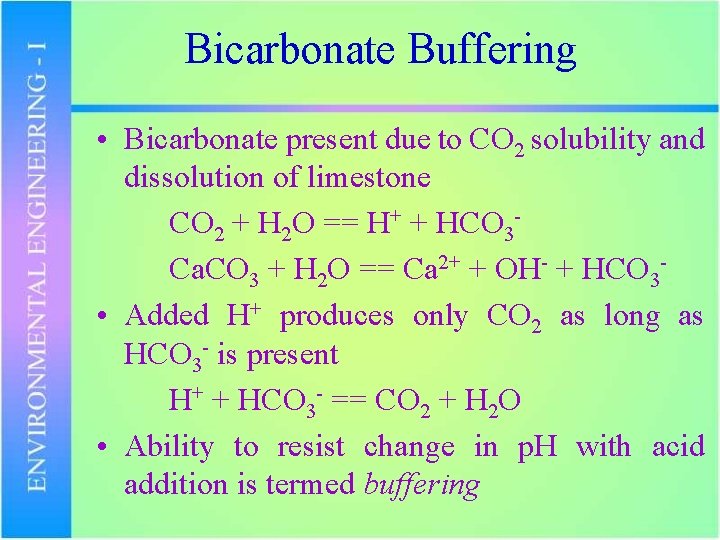
Bicarbonate Buffering • Bicarbonate present due to CO 2 solubility and dissolution of limestone CO 2 + H 2 O == H+ + HCO 3 Ca. CO 3 + H 2 O == Ca 2+ + OH- + HCO 3 • Added H+ produces only CO 2 as long as HCO 3 - is present H+ + HCO 3 - == CO 2 + H 2 O • Ability to resist change in p. H with acid addition is termed buffering

Implications • Critical Issue is the amount of calcite in the watershed • Particular problem in New England – large amount of sulfur are released in midwest – bedrock is largely granite – soils are shallow • Many lakes are unproductive due to low p. H

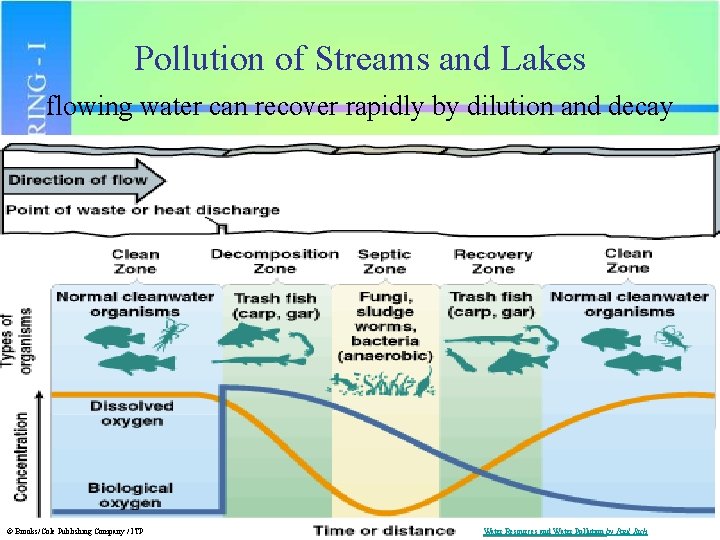
Pollution of Streams and Lakes flowing water can recover rapidly by dilution and decay © Brooks/Cole Publishing Company / ITP Water Resources and Water Pollution by Paul Rich

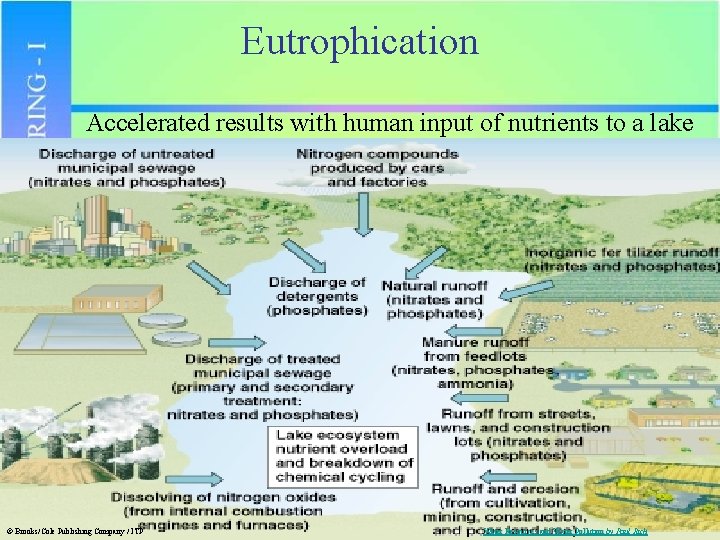
Eutrophication Accelerated results with human input of nutrients to a lake © Brooks/Cole Publishing Company / ITP Water Resources and Water Pollution by Paul Rich