Science Joke of the Day Two atoms were









- Slides: 20

Science Joke of the Day Two atoms were walking on the street. One atom lost an electron. The second atom asks: Are you sure you want to continue walking? Yes, I’m positive.

Review from last day: Write the names of the following compounds. 1. 2. 3. 4. 5. Ca. F 2 KBr Be. O Li. Cl Rb 3 P

Review from last day: Write the names of the following compounds. 1) Ca. F 2 Calcium fluoride 2) KBr Potassium bromide 3) Be. O Beryllium oxide 4) Li. Cl Lithium chloride 5) Rb 3 P Rubidium phosphide

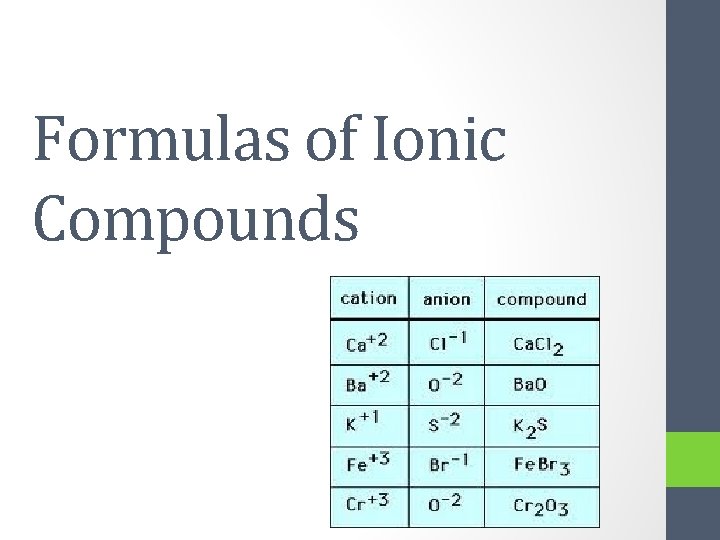
Formulas of Ionic Compounds

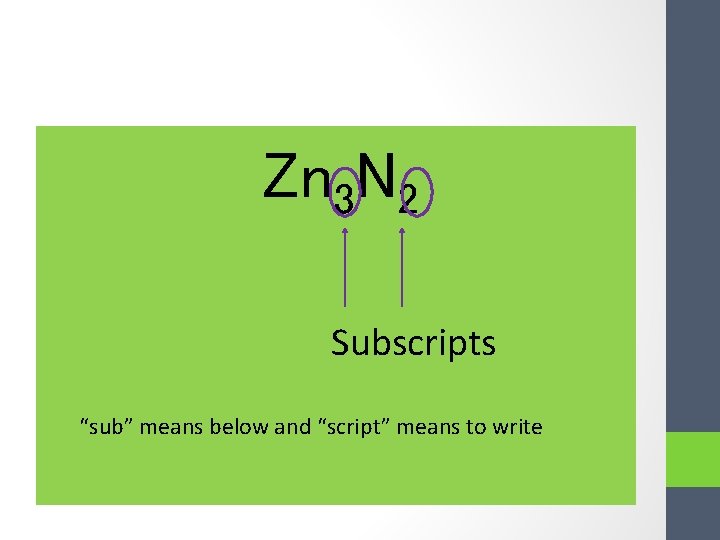
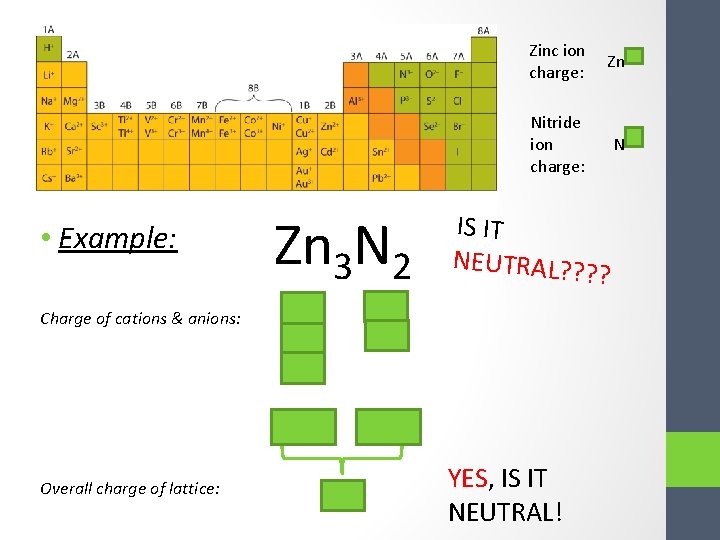
Zn 3 N 2 Subscripts “sub” means below and “script” means to write

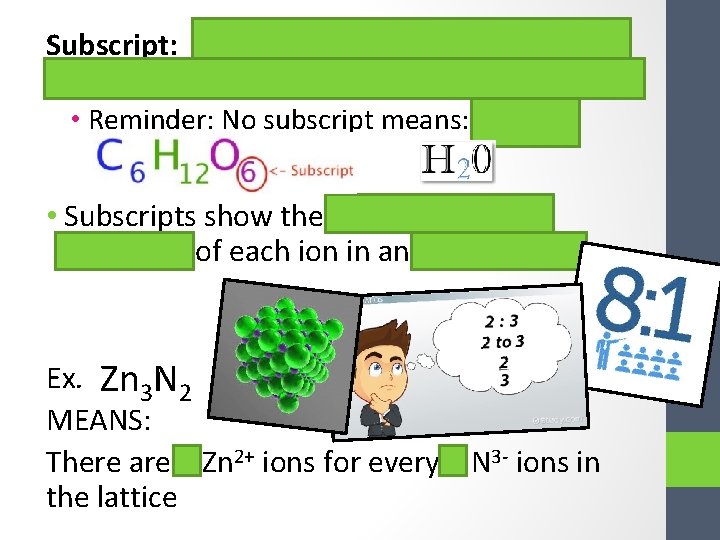
Subscript: the tiny number located on the bottom right of each element in a compound • Reminder: No subscript means: 1! • Subscripts show the RATIO (relative amounts) of each ion in an ionic lattice Ex. Zn 3 N 2 MEANS: There are 3 Zn 2+ ions for every 2 N 3 - ions in the lattice

SUBSCR IPTS = ?

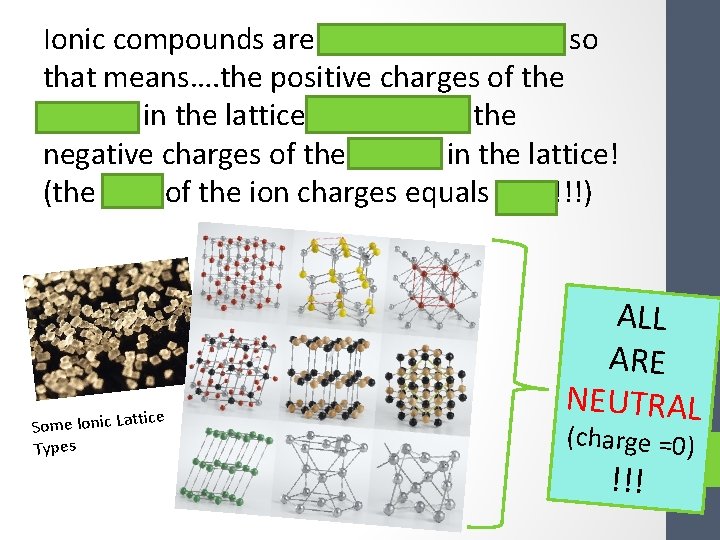
Ionic compounds are electrically neutral so that means…. the positive charges of the cations in the lattice balance out the negative charges of the anions in the lattice! (the sum of the ion charges equals zero!!!) ttice Some Ionic La Types ALL ARE NEUTRAL (charge =0) !!!

Zinc ion Zn 2+ charge: Nitride ion charge: • Example: Charge of cations & anions: Zn 3 N 2 Zn 2+ N 3 - IS IT NEUTRAL? ? ? ? Zn 2+ Overall charge of lattice: ___ 6+ 60!! N 3 - YES, IS IT NEUTRAL!

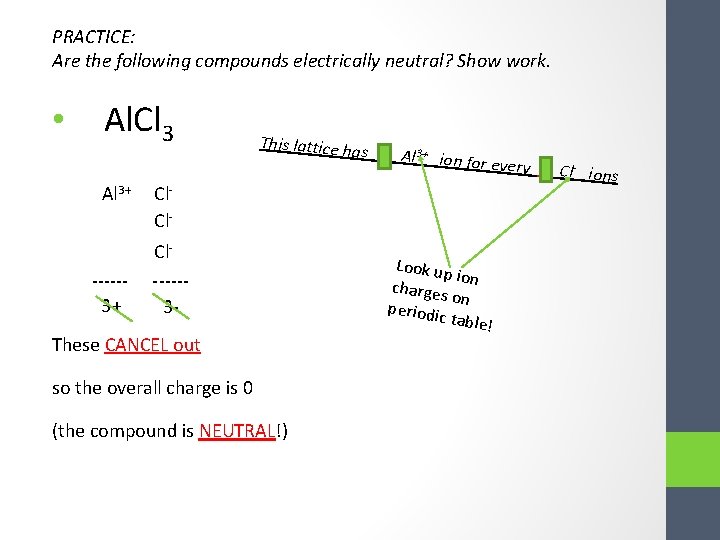
PRACTICE: Are the following compounds electrically neutral? Show work. • Al. Cl 3 Al 3+ -----3+ This lattice ha Cl. Cl-----3 - These CANCEL out so the overall charge is 0 (the compound is NEUTRAL!) s 1 Al 3+ ion for every 3 C l ions Look up io charges n o periodi n c table !

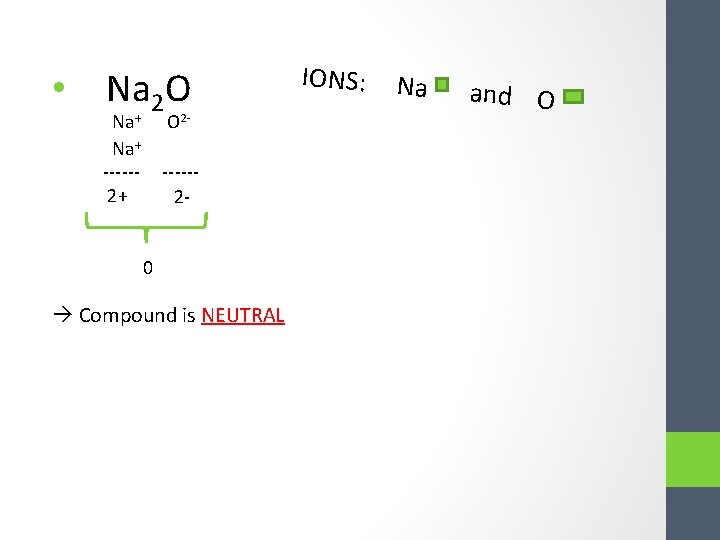
• Na 2 O Na+ -----2+ O 2 - -----2 - 0 Compound is NEUTRAL IONS: Na + and O 2 -

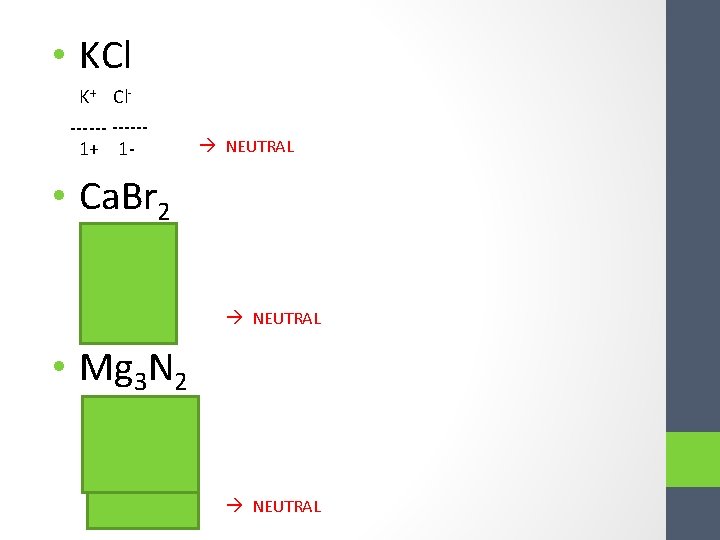
• KCl K+ Cl------1+ 1 - NEUTRAL • Ca. Br 2 Ca 2+ Br ----- 2+ 2 - NEUTRAL • Mg 3 N 2 Mg 2+ N 3 - Mg 2+ ----- 6+ 6 - NEUTRAL

Can you work backwards and figure out what the subscripts of the formula are? 1. magnesium oxide 2. Zirconium sulfide 3. Aluminum fluoride

Short Cut • “Swap and Drop” • https: //www. youtube. com/watch? v=Zem. NCgv. M 3 Kw

Rules for writing formulas of ionic compounds: 1. Identify each ion and its charge 2. Swap and Drop the “charges” into “subscripts” 3. Double check to make sure that the final formula has the smallest whole number ratio

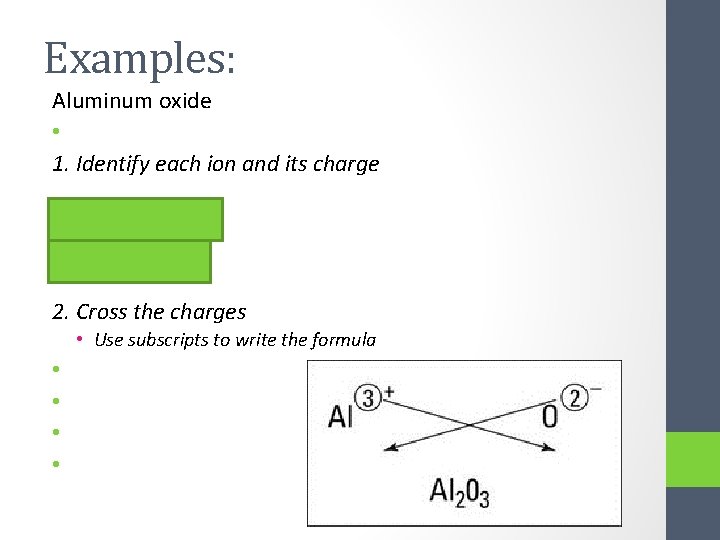
Examples: Aluminum oxide • 1. Identify each ion and its charge • Cation = Al 3+ • Anion = O 22. Cross the charges • Use subscripts to write the formula • •

3. Double check to make sure that the final formula has the smallest whole number ratio • Al 2 O 3

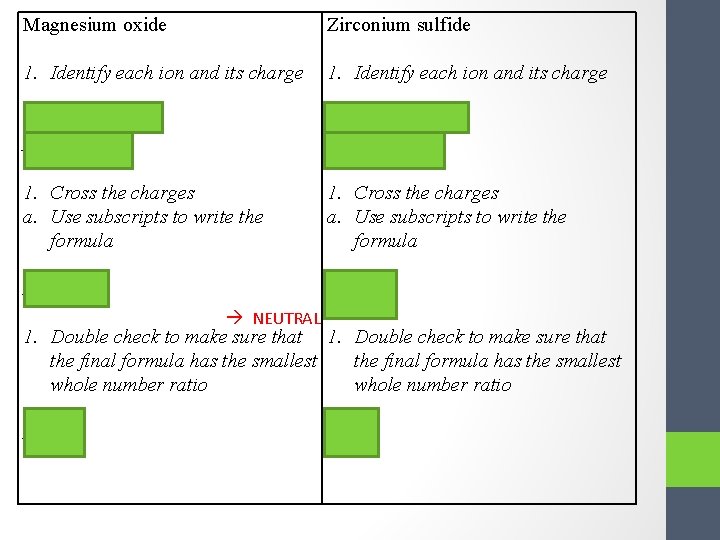
Magnesium oxide Zirconium sulfide 1. Identify each ion and its charge Cation = Mg 2+ Cation = Zr 4+ Anion = O 2 - Anion = S 2 - 1. Cross the charges a. Use subscripts to write the formula Mg 2 O 2 Zr 2 S 4 NEUTRAL 1. Double check to make sure that the final formula has the smallest whole number ratio Mg. O Zr. S 2


Try practice problems on pg. 87 of your text (Make sure the ones on pg. 86 are done!)
 Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Day 1 day 2 day 817
Day 1 day 2 day 817 What is my favourite subject
What is my favourite subject Two witches were watching two watches
Two witches were watching two watches Kesler science atoms answer key
Kesler science atoms answer key Vibrations of crystals with monatomic basis
Vibrations of crystals with monatomic basis What forms when two atoms combine?
What forms when two atoms combine? The force that holds two atoms together
The force that holds two atoms together Future in the past was were going to examples
Future in the past was were going to examples The tater family
The tater family Titration jokes
Titration jokes Joke klaassen
Joke klaassen Schrodinger joke
Schrodinger joke Panda eats, shoots and leaves joke
Panda eats, shoots and leaves joke Amino acid joke
Amino acid joke Anion joke
Anion joke Joke cycles
Joke cycles Joke cycles
Joke cycles Inflation
Inflation Ketchup joke pulp fiction
Ketchup joke pulp fiction