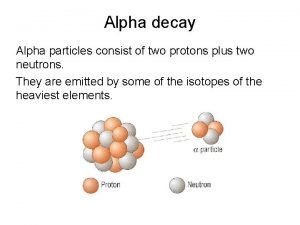
Radioactive Decays Transformation Kinetic HalfLife Different radioactive atoms




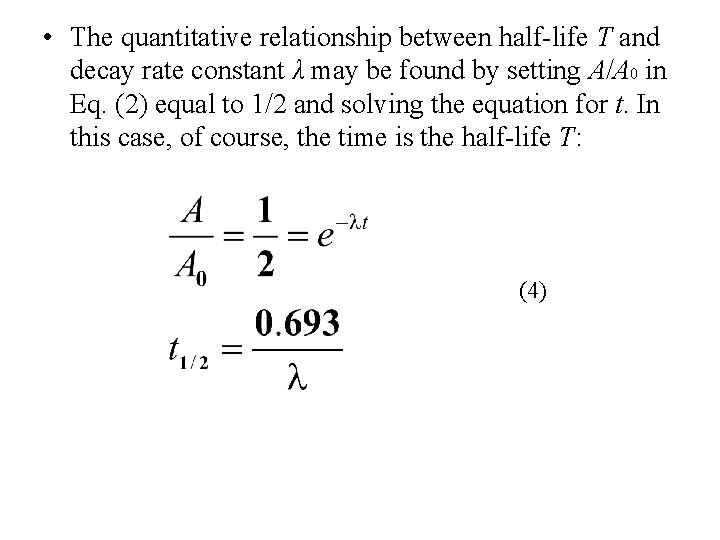


- Slides: 19

Radioactive Decays Transformation Kinetic

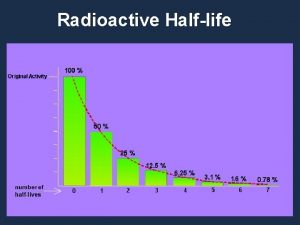
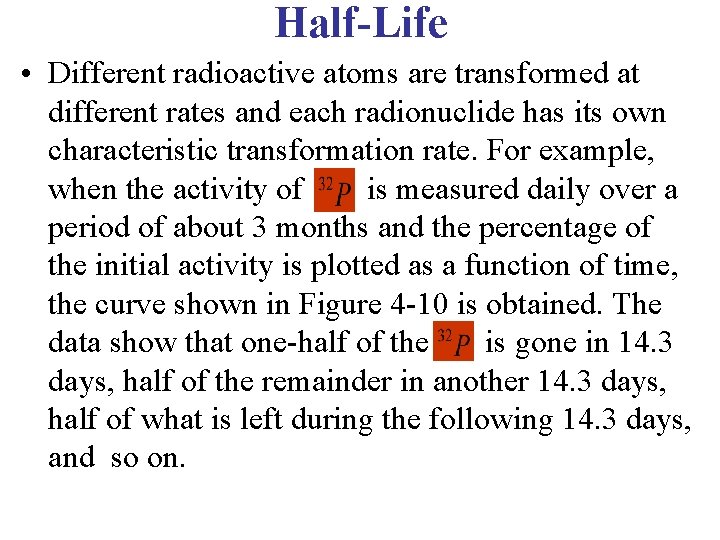
Half-Life • Different radioactive atoms are transformed at different rates and each radionuclide has its own characteristic transformation rate. For example, when the activity of is measured daily over a period of about 3 months and the percentage of the initial activity is plotted as a function of time, the curve shown in Figure 4 -10 is obtained. The data show that one-half of the is gone in 14. 3 days, half of the remainder in another 14. 3 days, half of what is left during the following 14. 3 days, and so on.


• The time required for any given radionuclide to decrease to one-half of its original quantity is a measure of the speed with which it undergoes radioactive transformation. This period of time is called the half-life and is characteristic of the particular radionuclide. • Each radionuclide has its own unique rate of transformation, and no operation, either chemical or physical, is known that will change the transformation rate; the decay rate of a radionuclide is an unalterable property of that nuclide. • Half-lives of radionuclides range from microseconds to billions of years.

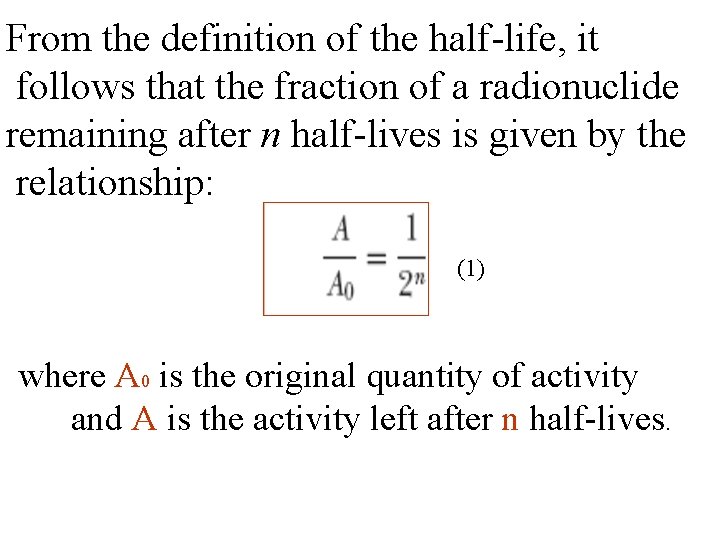
From the definition of the half-life, it follows that the fraction of a radionuclide remaining after n half-lives is given by the relationship: (1) where A 0 is the original quantity of activity and A is the activity left after n half-lives.

• If the decay data for any radionucide are plotted on semilog paper, with the activity measurements recorded on the logarithmic axis and time on the linear axis, a straight line is obtained. If time is measured in units of half-lives, the straight line shown in the following Figure is obtained.


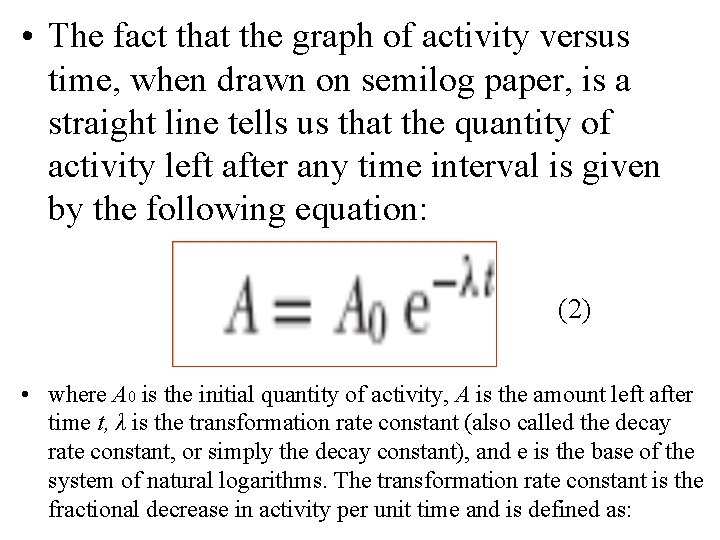
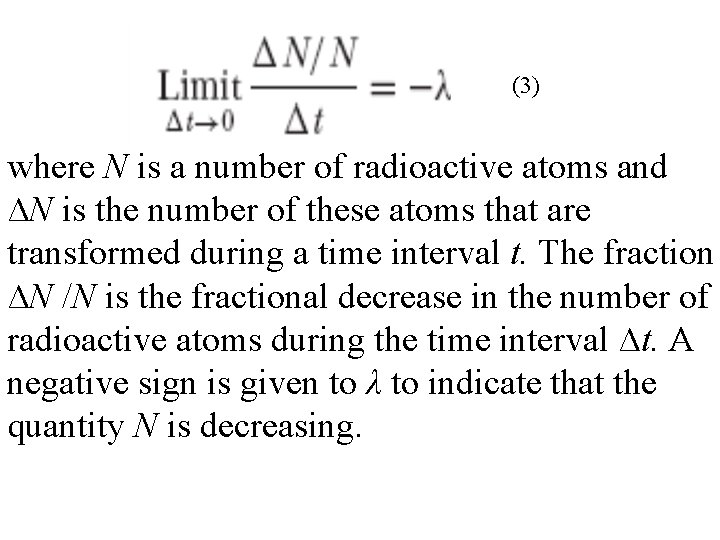
• The fact that the graph of activity versus time, when drawn on semilog paper, is a straight line tells us that the quantity of activity left after any time interval is given by the following equation: (2) • where A 0 is the initial quantity of activity, A is the amount left after time t, λ is the transformation rate constant (also called the decay rate constant, or simply the decay constant), and e is the base of the system of natural logarithms. The transformation rate constant is the fractional decrease in activity per unit time and is defined as:

(3) where N is a number of radioactive atoms and ∆N is the number of these atoms that are transformed during a time interval t. The fraction ∆N /N is the fractional decrease in the number of radioactive atoms during the time interval ∆t. A negative sign is given to λ to indicate that the quantity N is decreasing.
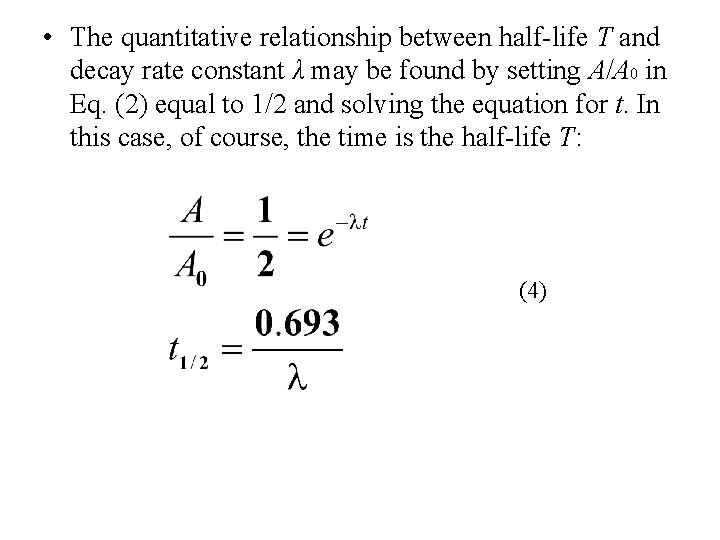
• The quantitative relationship between half-life T and decay rate constant λ may be found by setting A/A 0 in Eq. (2) equal to 1/2 and solving the equation for t. In this case, of course, the time is the half-life T: (4)

Average Life Although the half-life of a particular radionuclide is a unique, reproducible characteristic of that nuclide, it is nevertheless a statistical property and is valid only because of the very large number of atoms involved. (One microgram of radium contains Any particular atom of a radionuclide may be transformed at any time, from zero to infinity. For some applications, as in the case of dosimetry of internally deposited radioactive material (to be discussed later), it is convenient to use the average life of the radioisotope. The average life is defined simply as the: sum of the lifetimes of the individual atoms divided by the total number of atoms originally present.

(5) where N 0 is the number of radioactive atoms in existence at time t = 0. Since we have This expression, when integrated by parts by letting t=u and λ e(-λt)= dv, shows the value for the mean life of a radioisotope to be: (7)

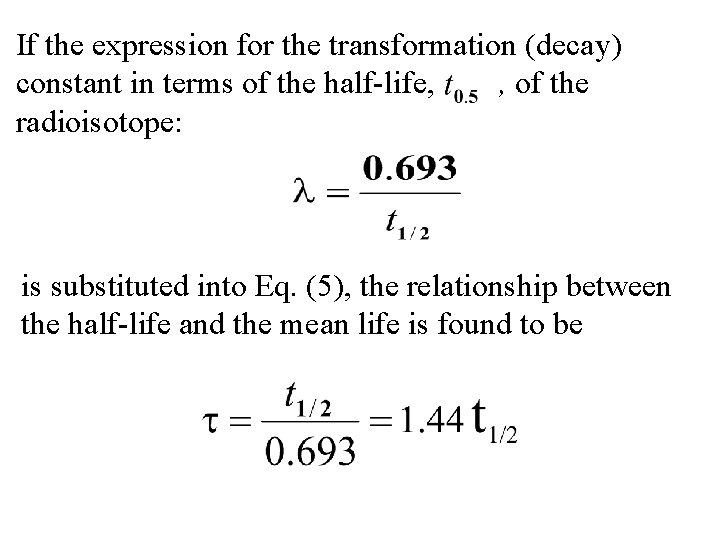
If the expression for the transformation (decay) constant in terms of the half-life, , of the radioisotope: is substituted into Eq. (5), the relationship between the half-life and the mean life is found to be

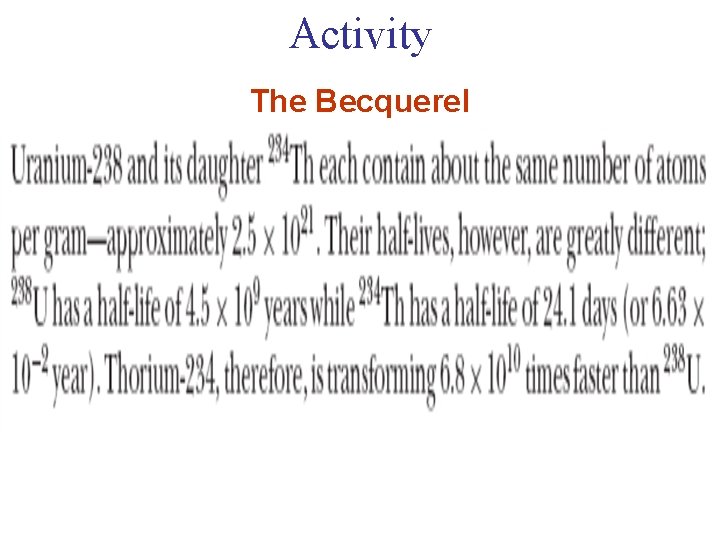
Activity The Becquerel

• The unit for quantity of radioactivity must be based on the number of radioactive decays occurring within a prescribed time in the radioactive material. Activity is defined as “the number of decays within a given”. Two units for measuring the activity are used. The SI unit is called the Becquerel, symbolized by Bq, and is defined as “that quantity of radioactive material in which one atom is transformed per second (tps)”. Very often, the term disintegration is used instead of transformation and the Becquerel is defined in term of disintegrations per second, dps. 1 Bq = 1 tps = 1 dps

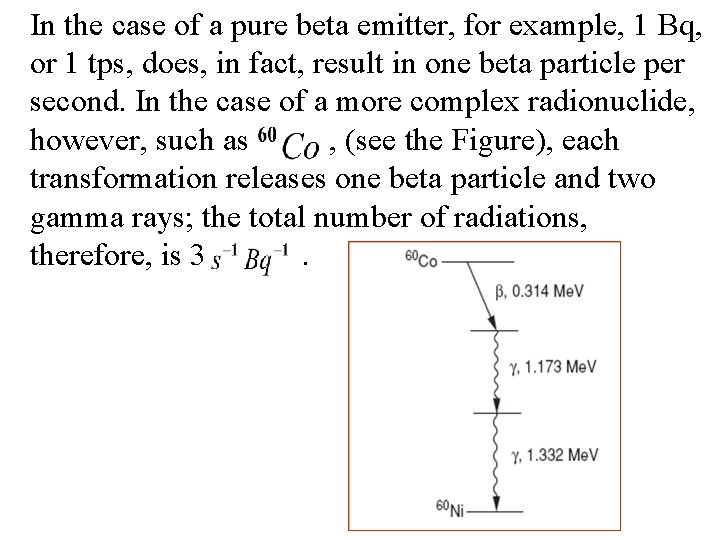
In the case of a pure beta emitter, for example, 1 Bq, or 1 tps, does, in fact, result in one beta particle per second. In the case of a more complex radionuclide, however, such as , (see the Figure), each transformation releases one beta particle and two gamma rays; the total number of radiations, therefore, is 3.

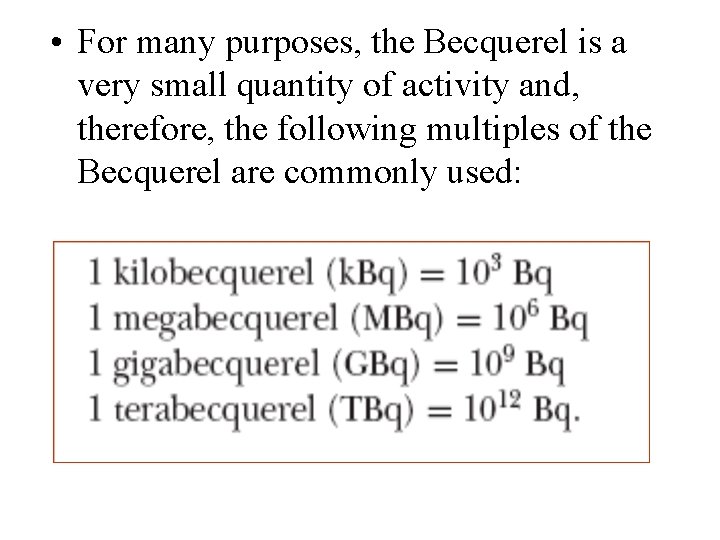
• For many purposes, the Becquerel is a very small quantity of activity and, therefore, the following multiples of the Becquerel are commonly used:

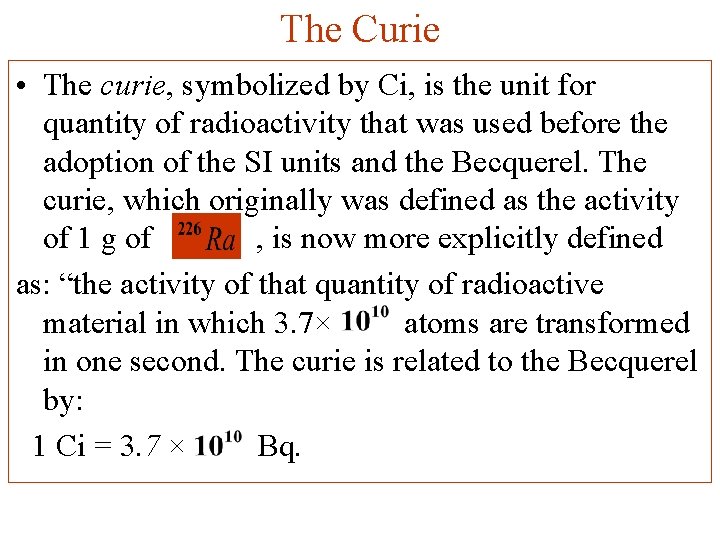
The Curie • The curie, symbolized by Ci, is the unit for quantity of radioactivity that was used before the adoption of the SI units and the Becquerel. The curie, which originally was defined as the activity of 1 g of , is now more explicitly defined as: “the activity of that quantity of radioactive material in which 3. 7× atoms are transformed in one second. The curie is related to the Becquerel by: 1 Ci = 3. 7 × Bq.

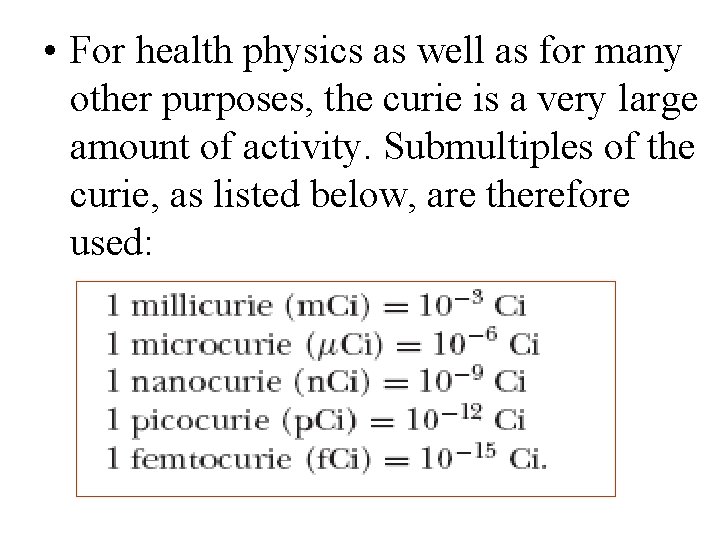
• For health physics as well as for many other purposes, the curie is a very large amount of activity. Submultiples of the curie, as listed below, are therefore used:
 Plutonium half life
Plutonium half life Why do different atoms produce different colors
Why do different atoms produce different colors Plutonium-239 alpha decay equation
Plutonium-239 alpha decay equation Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Regents periodic table
Regents periodic table Acids and bases song
Acids and bases song Library.thinkquest.org 19537
Library.thinkquest.org 19537 Manufactured boards examples
Manufactured boards examples Different culture have different moral codes
Different culture have different moral codes Different people different things
Different people different things Thermosoftening plastics examples
Thermosoftening plastics examples Different angle different story
Different angle different story Argumenterande tal struktur
Argumenterande tal struktur Sound will travel at different speeds in different mediums.
Sound will travel at different speeds in different mediums. Radioactive
Radioactive Discrete radioactive particles
Discrete radioactive particles Beta minus decay
Beta minus decay Radioactive tracers in agriculture
Radioactive tracers in agriculture Conclusion for radioactive pollution
Conclusion for radioactive pollution Radioactive decay formula
Radioactive decay formula