Pyrethroid Insecticides Pyrethroid Insecticides Derived from natural product









- Slides: 22

Pyrethroid Insecticides

Pyrethroid Insecticides • Derived from natural product -– Pyrethrum – Found in Chrysanthemum cinerarifolium • Synthetic pyrethroids – Variable in structure, toxicity – Generally benign environmentally • most are highly toxic to fish • Major uses in – Home and garden – Agriculture – Medical entomology

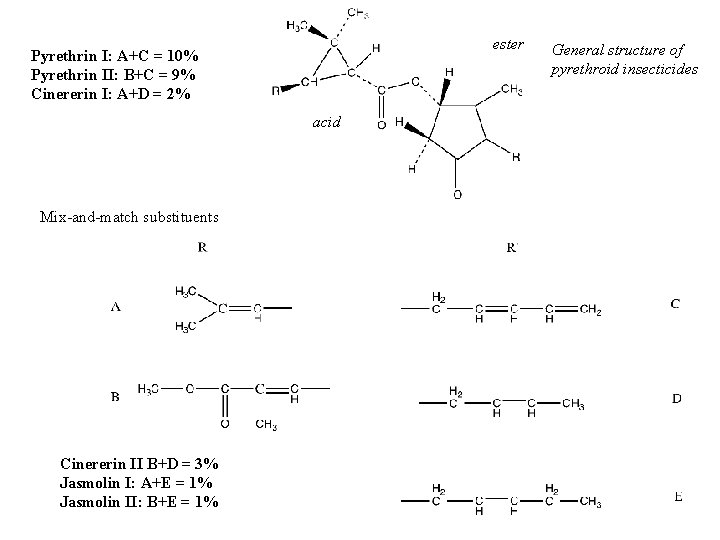
Natural Pyrethrum • Mixture of esters: – Chrysanthemic acid or pyrethric acid – + pyrethrolone, cinerolone, and/or jasmololone • Structural features essential for insecticidal activity: – 3 -C ring • Variables: – Enantiomers around asymmetric carbon atoms

ester Pyrethrin I: A+C = 10% Pyrethrin II: B+C = 9% Cinererin I: A+D = 2% acid Mix-and-match substituents Cinererin II B+D = 3% Jasmolin I: A+E = 1% Jasmolin II: B+E = 1% General structure of pyrethroid insecticides

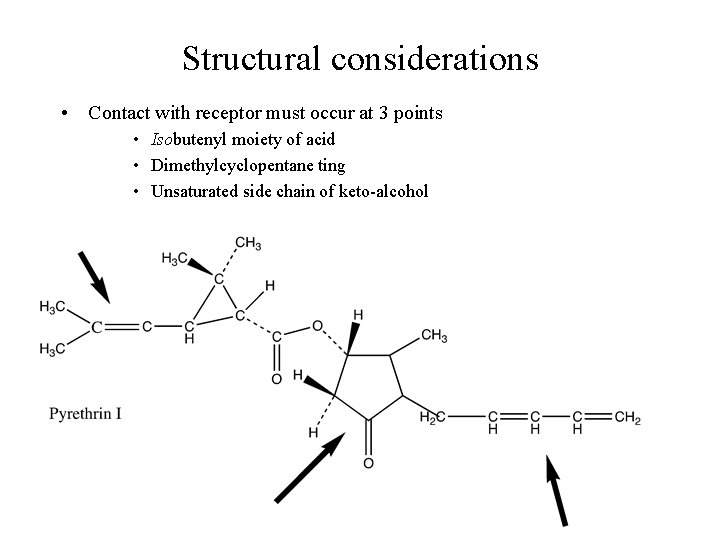
Structural considerations • Contact with receptor must occur at 3 points • Isobutenyl moiety of acid • Dimethylcyclopentane ting • Unsaturated side chain of keto-alcohol


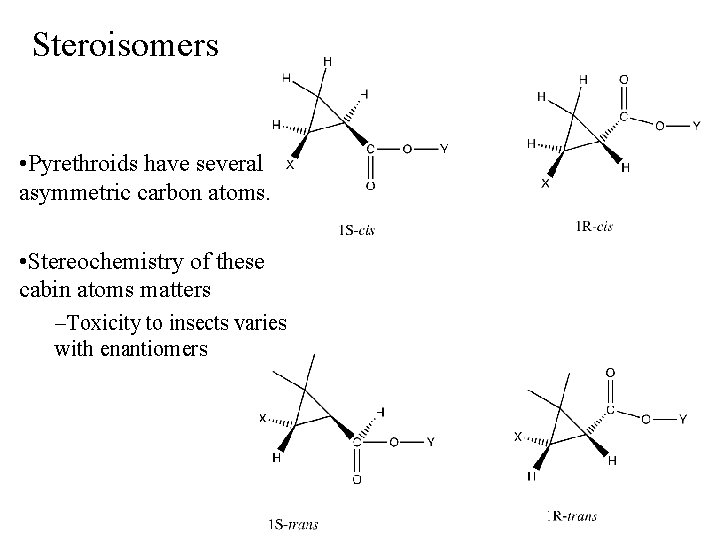
Steroisomers • Pyrethroids have several asymmetric carbon atoms. • Stereochemistry of these cabin atoms matters –Toxicity to insects varies with enantiomers

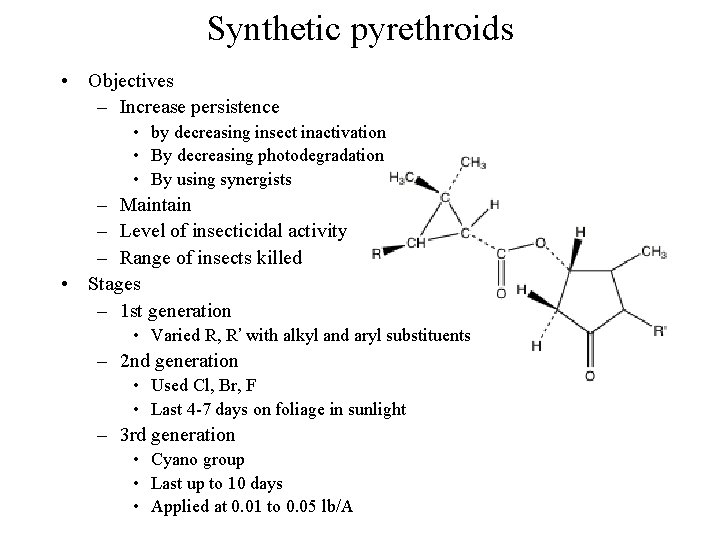
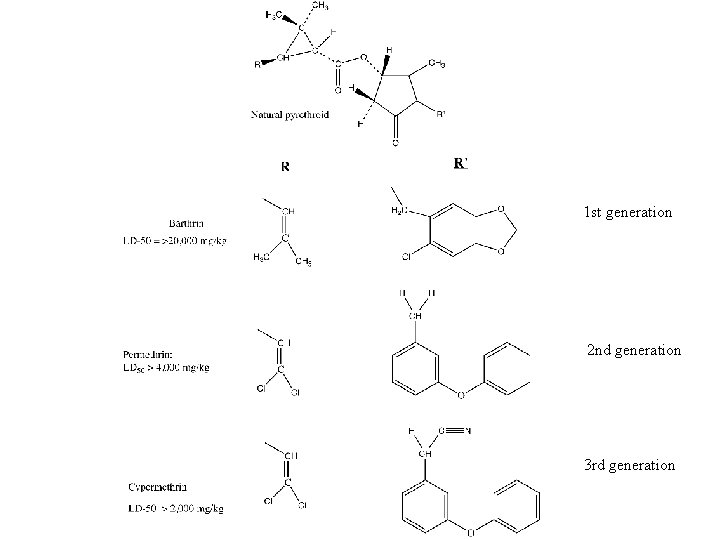
Synthetic pyrethroids • Objectives – Increase persistence • by decreasing insect inactivation • By decreasing photodegradation • By using synergists – Maintain – Level of insecticidal activity – Range of insects killed • Stages – 1 st generation • Varied R, R’ with alkyl and aryl substituents – 2 nd generation • Used Cl, Br, F • Last 4 -7 days on foliage in sunlight – 3 rd generation • Cyano group • Last up to 10 days • Applied at 0. 01 to 0. 05 lb/A

1 st generation 2 nd generation 3 rd generation

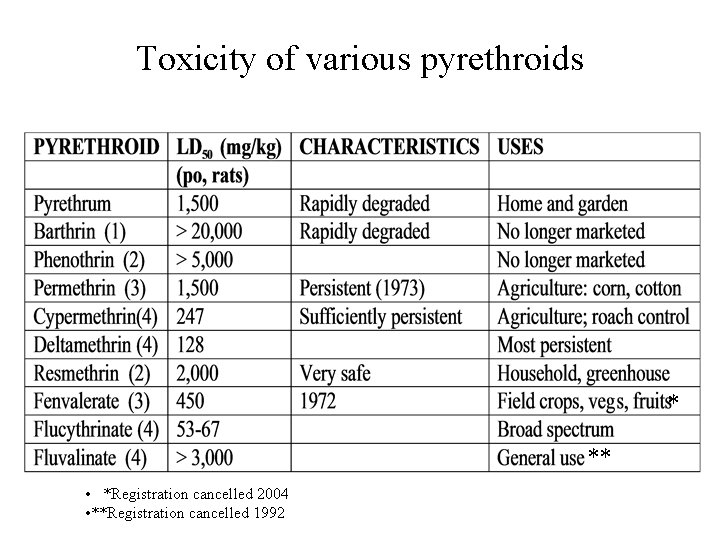
Toxicity of various pyrethroids * ** • *Registration cancelled 2004 • **Registration cancelled 1992

Systemic toxicity: Type I • Pyrethroids without cyano group • Target – CNS, • primarily brain stem • Cerebellum and cerebrum not primary targets – Progressive development of fine whole body tremor – Exaggerated startle reflex • • • Large increase in metabolic rate Uncoordinated twitching Hyperexcitability Hyperthermia Death results from metabolic exhaustion and hyperthermia

Systemic toxicity: Type II • Pyrethroids with cyano group • Target: – CNS – All regions affected • Symptoms complex – – – Salivation Rolling gait - increased extensor tone in hind limbs Spasms due to sensory stimuli Tonic seizures Apnea death

Allergic Reactions • Of topical exposure – Contact dermatitis • Either natural or synthetic pyrethroids – Irritant effect • Not inflammatory response • Lasts up to 24 hours • May include numbness or parasthesias – “Annoying but not disabling” – Apparently completely reversible • Systemic allergic responses – Pyrethroids derive from chrysanthemum components – Allergies are well known to occur • • Respiratory May be serious Rarely, fatal Occupational exposure ---> emphysema (rare? )

Cellular Toxicity • • Insecticidal activity: – Prolong opening of voltage-gated sodium channels Mammalian toxicity – Sodium channels • Variable, depending on isoform – Some voltage-gated calcium channels – Some voltage-gated chloride channels – Peripheral-type benzodiazepene receptors • Contributing to convulsive effects • Variations of effects on ion channels: – Pyrethroids have high affinity for active membrane Na+ channels • Only affect open channels, blocking them – “Open channel blockers” – Pyrethroids without alpha-cyano group • Cause nerve channels to close very slowly – Pyrethroids with alpha-cyano group (4 th generation) • Cause delayed closure of Na channels

Transmembrane channels • • • Formed by proteins Hydrophilic channels through the lipid membrane May be permanently open May be gated: normally closed, open for cause Gates respond to • Ligands • Electrical charge • Gates may close rapidly after opening, even if signal is still being given • Example: neuromuscular junction – Electric nerve impulse reaches nerve terminal


Neuromuscular junction • Signal: electrical – Depolarization of nerve impulse – Decrease in membrane polarization opens voltage-gated Ca+ channels in presynaptic membrane – Ca+ ions stream into cell, triggering release of ACh • ACh receptors are ligand-gated – Transiently permeable to Na+ and K+ in presence of ACh


Gated transmembrane channels: presynaptic terminal – At terminal of axon: • • Depolarization opens voltage-gated Ca+2 channel Responds to nerve impulse Releases Ca+2 into axon terminal Causes ACh release into synaptic cleft

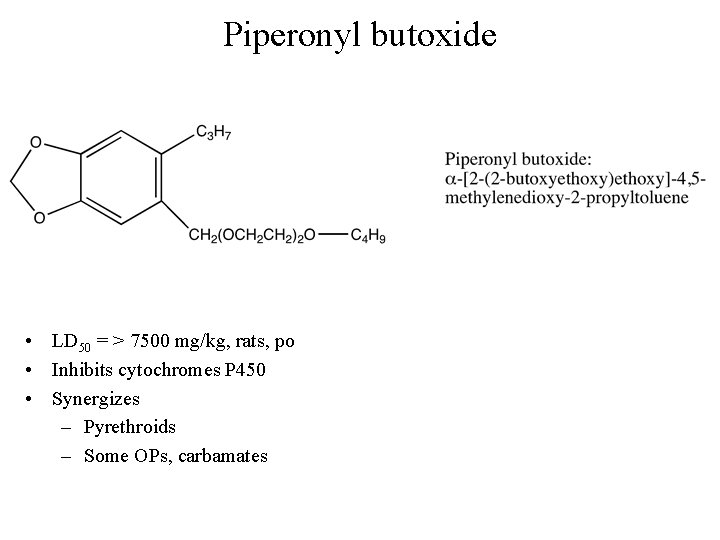
Degradation of pyrethroids • Photolytic – Very rapid for pyrethrum, early pyrethroids • Metabolic – – Extremely rapid for pyrethrum, early pyrethroids Less rapid for later generations of synthetics Mediated by P 450 s Inhibited by synergists • Piperonyl butoxide

Piperonyl butoxide • LD 50 = > 7500 mg/kg, rats, po • Inhibits cytochromes P 450 • Synergizes – Pyrethroids – Some OPs, carbamates

Ecotoxicology of Pyrethroids • Extremely toxic to fish • Mammalian toxicity – Minimal for most pyrethroids – Exceptions • Deltamethrin, 25 -60 mg/kg • Flucythrinate, 53 -87 mg/kg • Natural pyrethroids – Benign except for toxicity to fish • Synthetic pyrethroids – Increased persistence not of an order to raise concern about bioaccumulation – Movement into water from terrestrial applications is a danger with more persistent pyrethroids • Low application rates minimize this