Properties of Water Polarity Water is a polar






- Slides: 16

Properties of Water

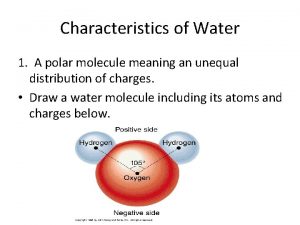
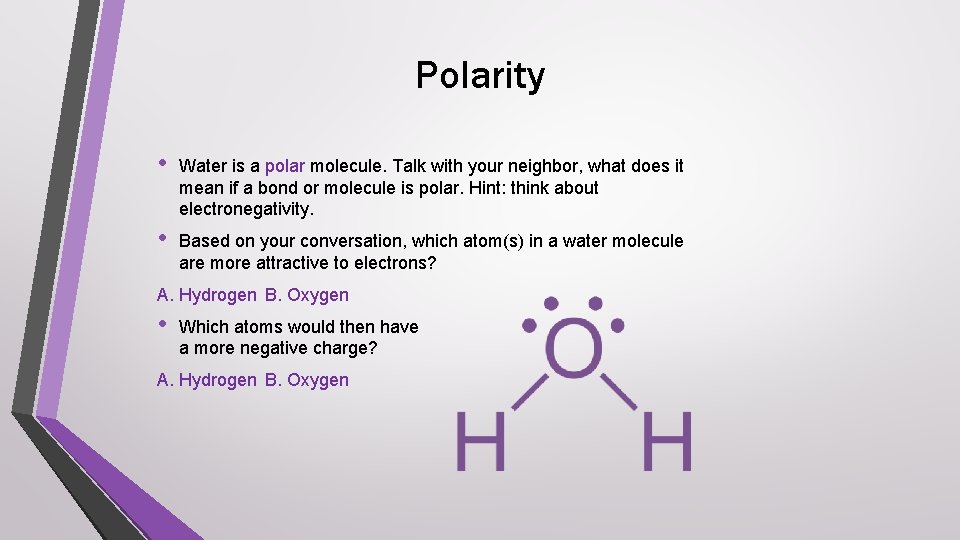
Polarity • Water is a polar molecule. Talk with your neighbor, what does it mean if a bond or molecule is polar. Hint: think about electronegativity. • Based on your conversation, which atom(s) in a water molecule are more attractive to electrons? A. Hydrogen B. Oxygen • Which atoms would then have a more negative charge? A. Hydrogen B. Oxygen

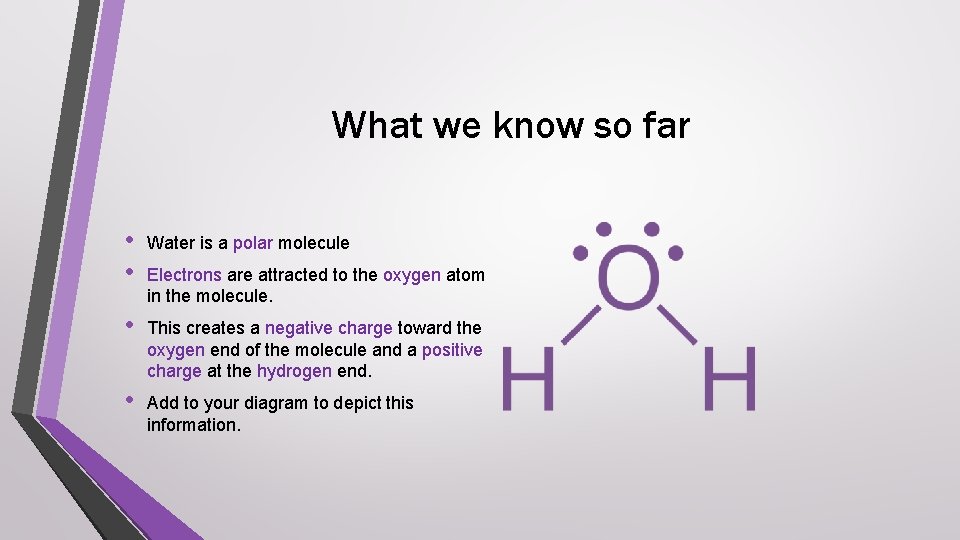
What we know so far • • Water is a polar molecule • This creates a negative charge toward the oxygen end of the molecule and a positive charge at the hydrogen end. • Add to your diagram to depict this information. Electrons are attracted to the oxygen atom in the molecule.

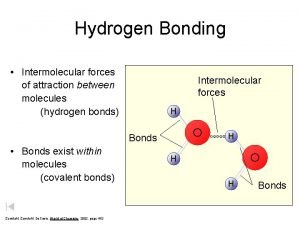
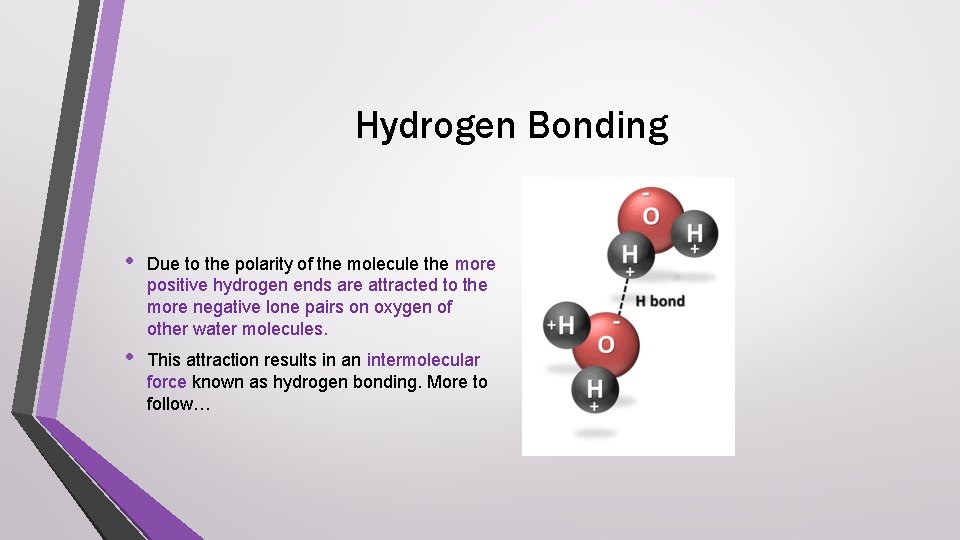
Hydrogen Bonding • Due to the polarity of the molecule the more positive hydrogen ends are attracted to the more negative lone pairs on oxygen of other water molecules. • This attraction results in an intermolecular force known as hydrogen bonding. More to follow…

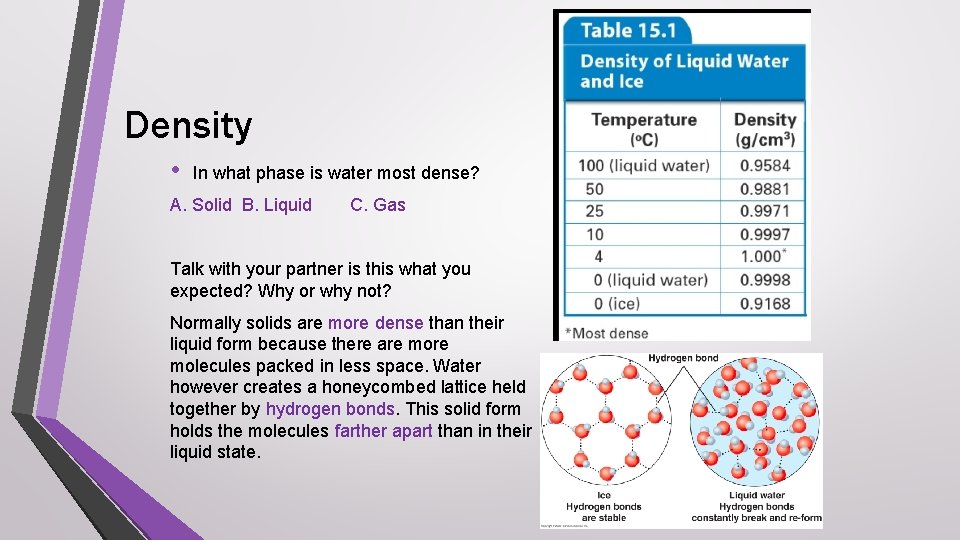
Density • In what phase is water most dense? A. Solid B. Liquid C. Gas Talk with your partner is this what you expected? Why or why not? Normally solids are more dense than their liquid form because there are molecules packed in less space. Water however creates a honeycombed lattice held together by hydrogen bonds. This solid form holds the molecules farther apart than in their liquid state.

Surface Tension • The inward force, or pull, that minimizes the surface area of a liquid is called surface tension. • The spherical shape of a water drop provides the minimum surface area for a give volume. This maximizes the ability of the molecules to interact. • Talk with your neighbor • How can you account for the high surface tension of water? • How does the addition of a surfactant impact water’s surface tension?

Vapor Pressure • The force exerted by a gas above a liquid in a sealed container is vapor pressure. • Water has unusually low vapor pressure, which means that it is difficult for water molecules to escape the surface of the liquid into the vapor phase. • Talk with your neighbor… • • Why would water’s vapor pressure be low? • Why is low vapor pressure of water important in nature? Why would heating water make vaporization occur more frequently?

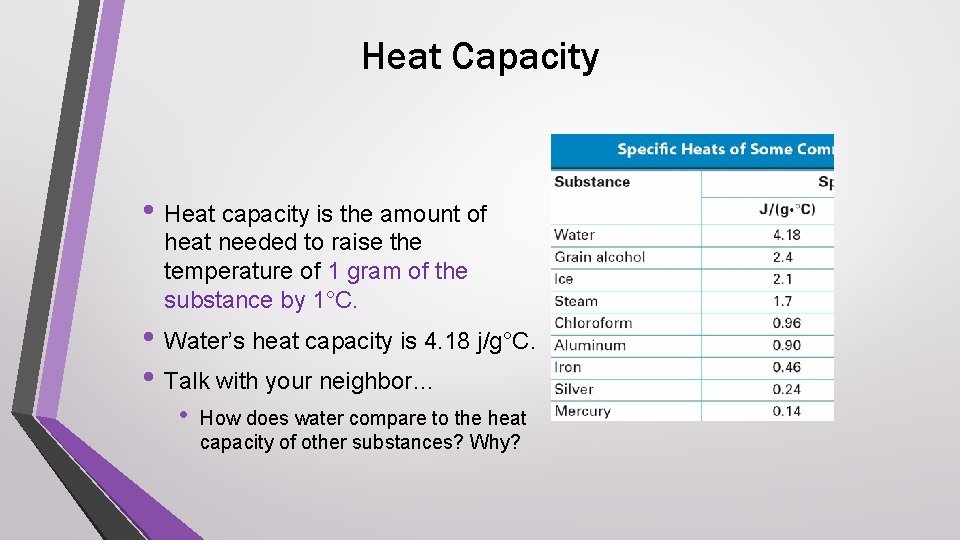
Heat Capacity • Heat capacity is the amount of heat needed to raise the temperature of 1 gram of the substance by 1°C. • Water’s heat capacity is 4. 18 j/g°C. • Talk with your neighbor… • How does water compare to the heat capacity of other substances? Why?

Intermolecular Forces (IMFs)

Intermolecular Forces • The forces with which molecules attract each other. • Intermolecular forces are weaker than ionic or covalent bonds. • Intermolecular forces are responsible for the physical state of a compound (solid, liquid or gas). • Types of IMFs: • • Weakest-Van der Waals: dipole-dipole & dispersion Strongest-Hydrogen Bonds

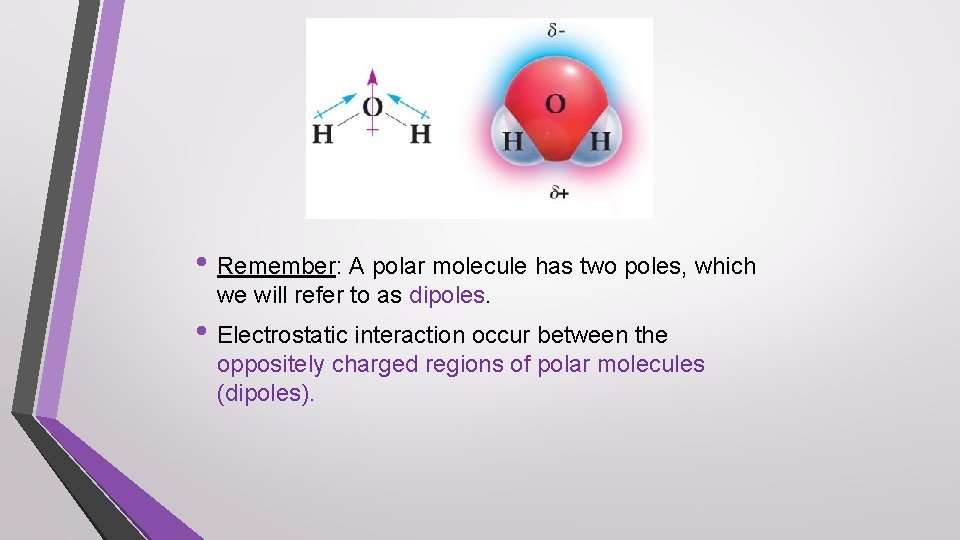
• Remember: A polar molecule has two poles, which we will refer to as dipoles. • Electrostatic interaction occur between the oppositely charged regions of polar molecules (dipoles).

Practice Question Which Molecules have Dipole Interactions? A) Polar B) Non-Polar Which of the following molecules has dipole interactions? A) F 2 B) CH 4 C) CH 3 Cl After each response tell your neighbor why you selected the answer you did.

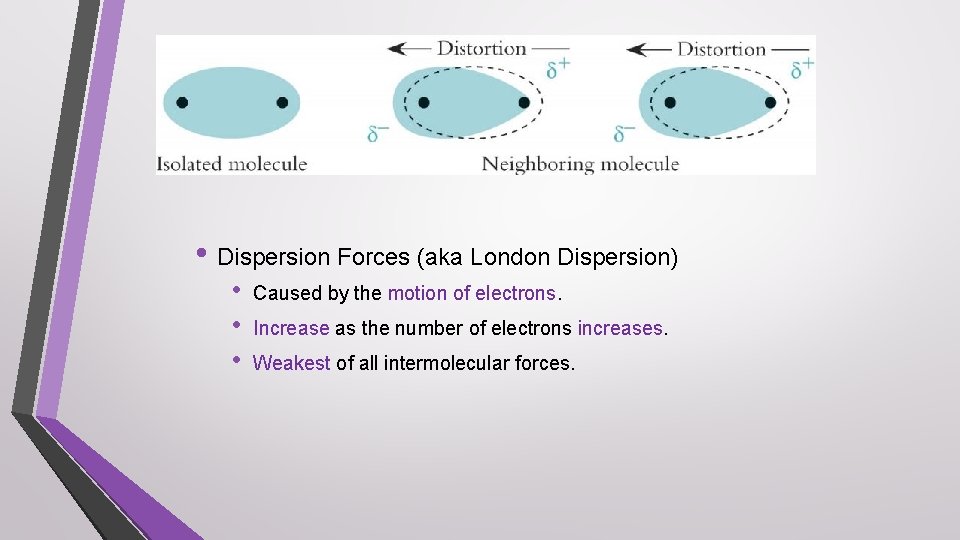
• Dispersion Forces (aka London Dispersion) • • • Caused by the motion of electrons. Increase as the number of electrons increases. Weakest of all intermolecular forces.

Hydrogen Bonding • Remember: Hydrogen bonding is the attraction between a hydrogen atom of a molecule to an unshared pair of electrons in another molecule. • Hydrogen bonding occurs in molecules where hydrogen is covalently bonded to a very electronegative element. • Hydrogen bonding occurs in molecules containing N, O, and F.

Hydrogen Bonding, Continued • Hydrogen bonds are the strongest of all intermolecular forces. • Hydrogen bonds are possible because in hydrogen atoms there is no shielding of the nucleus. • Talk with your neighbor… • What is nuclear shielding? • Hydrogen bonds are responsible for the physical properties of many biological substances and, more importantly, water.

Which of the following molecules can have hydrogen bonding? A) F 2 B) CH 4 C) H 2 O D) CH 3 Cl E) NH 3
 Nh3 polar or nonpolar
Nh3 polar or nonpolar Bond polarity vs molecular polarity
Bond polarity vs molecular polarity Polar properties of water
Polar properties of water Properties of water polarity
Properties of water polarity Water and water and water water
Water and water and water water Polar and nonpolar covalent bonds
Polar and nonpolar covalent bonds Enlace polar
Enlace polar Essential amino acids arginine
Essential amino acids arginine Polar polar attractions
Polar polar attractions What are polar and nonpolar dielectrics
What are polar and nonpolar dielectrics Qumica
Qumica Polarity of water
Polarity of water Is wax paper polar or nonpolar
Is wax paper polar or nonpolar Are water molecules polar
Are water molecules polar Describe the polar characteristics of a water molecule.
Describe the polar characteristics of a water molecule. Polar covalent bond in water
Polar covalent bond in water Zumdahl chemistry
Zumdahl chemistry