Properties of Water Polarity Polarity is when one









- Slides: 12

Properties of Water

Polarity Ø Polarity is when one atom attracts electrons more strongly than another atom. l In H 2 O, the oxygen atom (O) attracts electrons more than the hydrogen atom (H). Ø The water molecule acts like the oxygen is positively charged and the hydrogen is negatively charged.

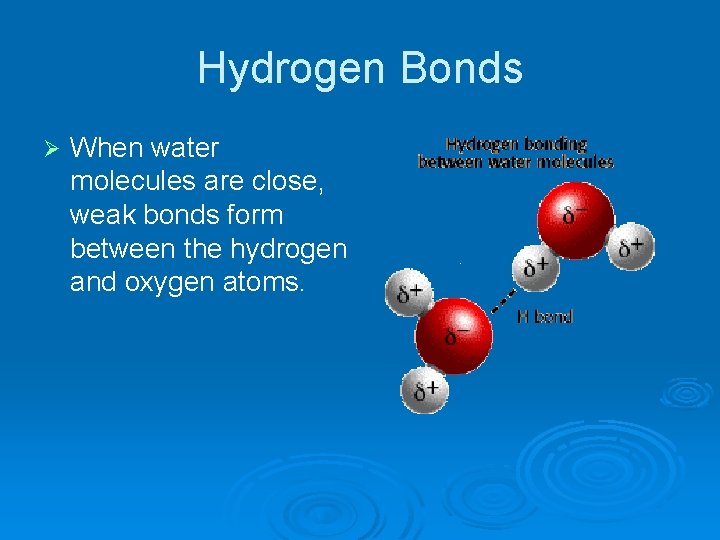
Hydrogen Bonds Ø When water molecules are close, weak bonds form between the hydrogen and oxygen atoms.

Hydrogen Bonds (con’t) Ø Cohesion – attraction between molecules of the same substance. l Because water forms hydrogen bonds, water molecules stick together very tightly • This is why water forms drops when placed on a flat surface.

Hydrogen Bonds (con’t) Ø Adhesion – attraction between molecules of different substances. l l Water can form hydrogen bonds with other materials. This is why water can rise up in narrow tubes.

Aqueous Mixtures Ø Water l l can form two kinds of mixtures. Solutions Suspensions

Solutions Ø All components of a solution are mixed evenly. l l Solute – the substance that is dissolved. Solvent – the substance that the solute dissolves in.

Suspensions Ø Small particles of material do not dissolve in water, but do not settle out. l Example: Coffee, Tea, Blood

p. H scale Ø Measure the amount of H+ ions in a solution. Ø Ranges between 0 -14. Ø For each increase in unit, the amount of H+ ions increases by 10. l l From p. H 2 to p. H 3, the p. H increases by 10 From p. H 2 to p. H 4, the p. H increases by 100

Acids Ø An acid is any substance that will form H+ ions in water. Ø Acids have a p. H range of less than 7

Bases Ø Bases are any substance that forms OHions in solution. Ø Bases have a p. H range over 7.

Class work Ø Pg l 57 1 -5, 11 -13, 15 -17
 Properties of water polarity
Properties of water polarity Properties of water polarity
Properties of water polarity Molecular shape for water
Molecular shape for water Molecular polarity vs bond polarity
Molecular polarity vs bond polarity Water and water and water water
Water and water and water water Waters chemical formula
Waters chemical formula One empire one god one emperor
One empire one god one emperor One one one little dog run
One one one little dog run One king one law one faith
One king one law one faith Byzantine definition
Byzantine definition Ford one plan
Ford one plan See one do one teach one
See one do one teach one See one, do one, teach one
See one, do one, teach one





















