Properties of water Polarity Water is a POLAR





- Slides: 8

Properties of water

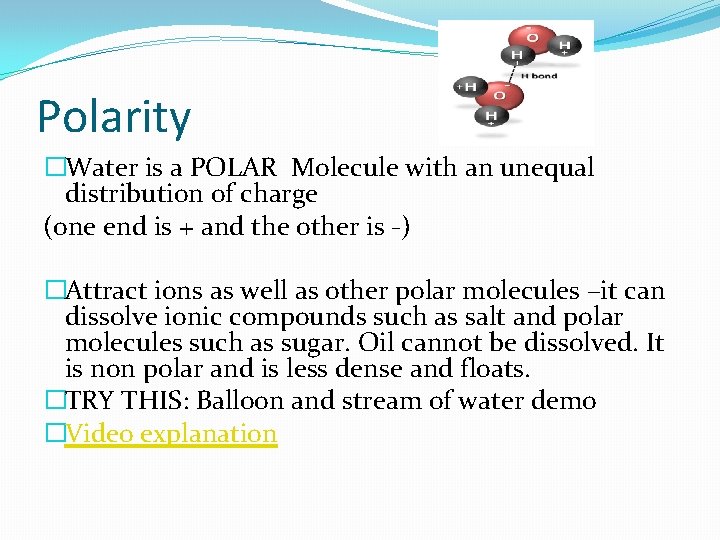
Polarity �Water is a POLAR Molecule with an unequal distribution of charge (one end is + and the other is -) �Attract ions as well as other polar molecules –it can dissolve ionic compounds such as salt and polar molecules such as sugar. Oil cannot be dissolved. It is non polar and is less dense and floats. �TRY THIS: Balloon and stream of water demo �Video explanation

Solvent �Water is called the "universal solvent" because it is capable of dissolving more substances than any other liquid. This is important to every living thing on earth. It means that wherever water goes, either through the air, the ground, or through our bodies, it takes along valuable chemicals, minerals, and nutrients. �Water becomes saturated when it contains so many dissolved substances, it no longer can dissolve any more. �Water can, however, become supersaturated (Having too many dissolved substances) �TRY THIS: Make a supersaturated solution of Borax and observe over a few days

Cohesion/Adhesion �Water molecules attract other water molecules (cohesion) and sticks to other molecules (adhesion) �Positive charge of hydrogen and attracts the negative charge oxygen of another water molecule �Causes water drops to form

Surface tension �Forms a film or a bead �This is why the water bug can float video �water in space (no gravity) �TRY THIS: How many drops of water can fit on a penny?

p. H �Water has a natural p. H of 7 (neutral). Since your body is mostly water your body also has a p. H around 7. �When substances are dissolved in water they can change the p. H to be more basic (8 -14 on a p. H scale) or more acidic (6 – 1 on a p. H scale). �Acid rain is an example click here �TRY THIS: test some common household substances for p. H. Environmental scientists test the p. H of soil and water collecting data to see the effects pollution may be having in an area.

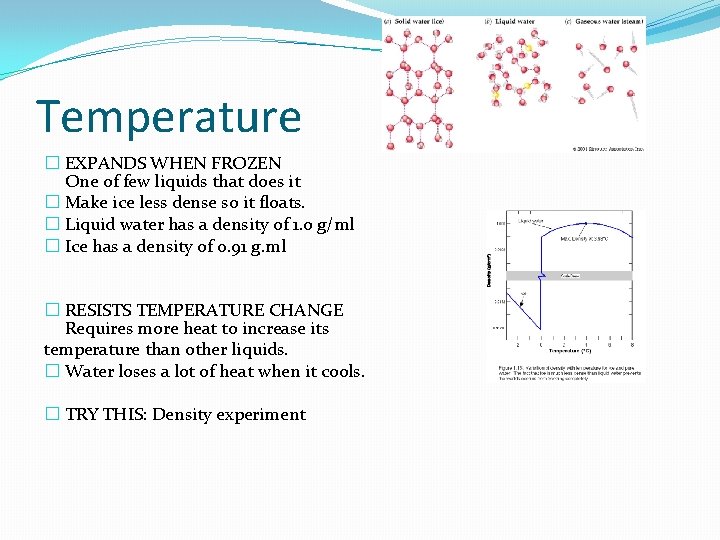
Temperature � EXPANDS WHEN FROZEN One of few liquids that does it � Make ice less dense so it floats. � Liquid water has a density of 1. o g/ml � Ice has a density of 0. 91 g. ml � RESISTS TEMPERATURE CHANGE Requires more heat to increase its temperature than other liquids. � Water loses a lot of heat when it cools. � TRY THIS: Density experiment

Water keeps temperatures form fluctuating too much. �The high heat capacity of water has a great deal to do with regulating extremes in the environment. For instance, our fish in the pond is indeed happy because the heat capacity of the water in her pond means the temperature of the water will stay relatively the same from day to night. �Specific Heat is the amount of heat that must be absorbed or lost for one gram of a substance to change its temperature by 1 o. C. �TRY THIS: Read specific heat pages in packet