Properties of Liquids Surface tension is the resistance

- Slides: 12


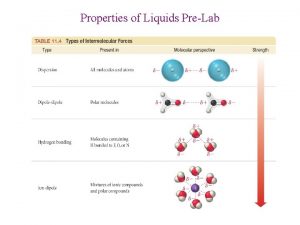
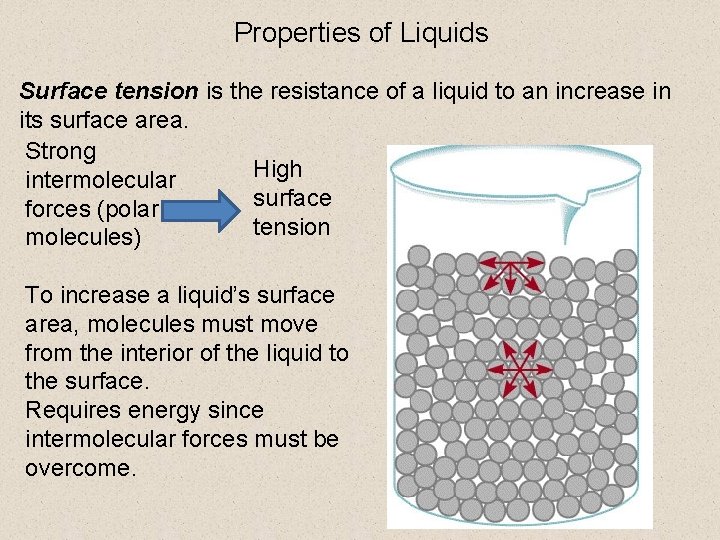
Properties of Liquids Surface tension is the resistance of a liquid to an increase in its surface area. Strong High intermolecular surface forces (polar tension molecules) To increase a liquid’s surface area, molecules must move from the interior of the liquid to the surface. Requires energy since intermolecular forces must be overcome.

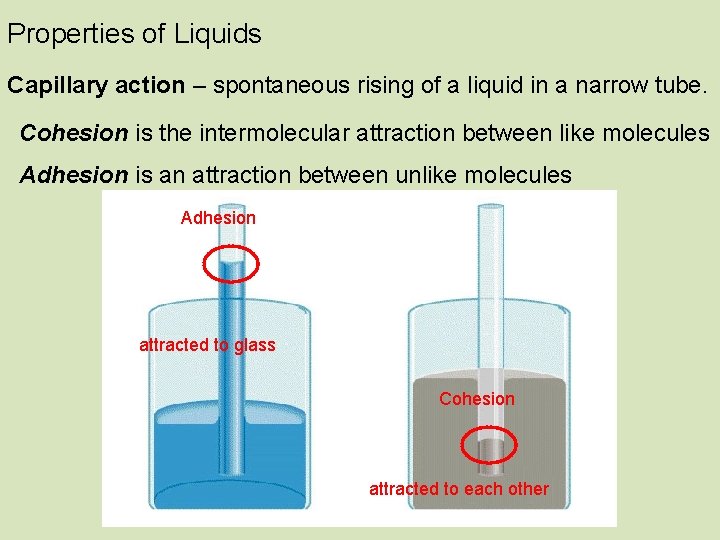
Properties of Liquids Capillary action – spontaneous rising of a liquid in a narrow tube. Cohesion is the intermolecular attraction between like molecules Adhesion is an attraction between unlike molecules Adhesion attracted to glass Cohesion attracted to each other

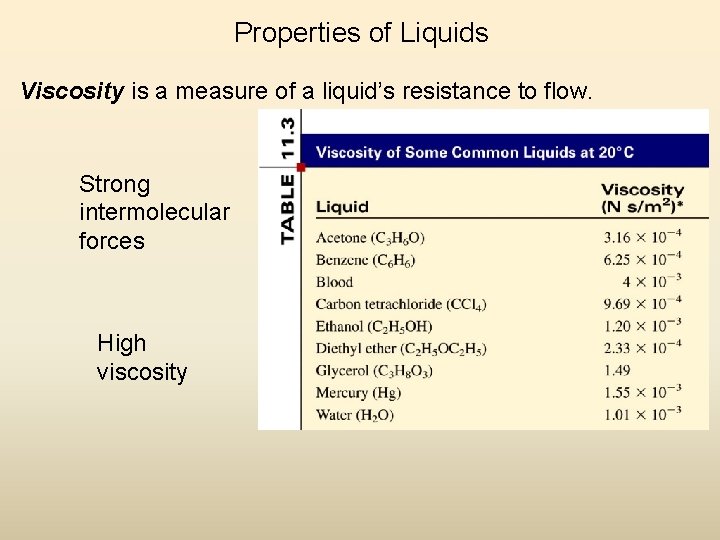
Properties of Liquids Viscosity is a measure of a liquid’s resistance to flow. Strong intermolecular forces High viscosity

Structures and Types of Solids

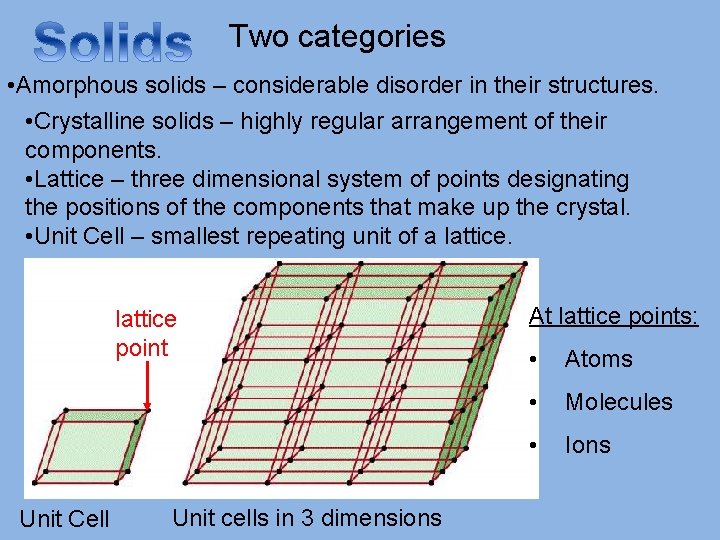
Two categories • Amorphous solids – considerable disorder in their structures. • Crystalline solids – highly regular arrangement of their components. • Lattice – three dimensional system of points designating the positions of the components that make up the crystal. • Unit Cell – smallest repeating unit of a lattice point Unit Cell Unit cells in 3 dimensions At lattice points: • Atoms • Molecules • Ions

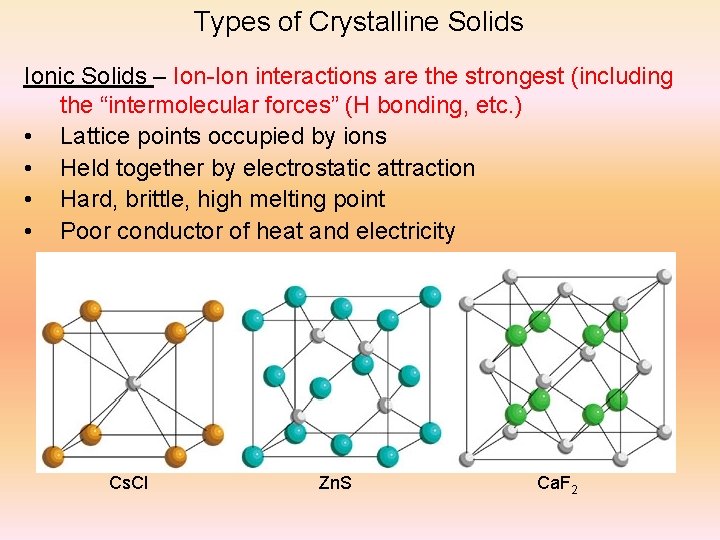
Types of Crystalline Solids Ionic Solids – Ion-Ion interactions are the strongest (including the “intermolecular forces” (H bonding, etc. ) • Lattice points occupied by ions • Held together by electrostatic attraction • Hard, brittle, high melting point • Poor conductor of heat and electricity Cs. Cl Zn. S Ca. F 2

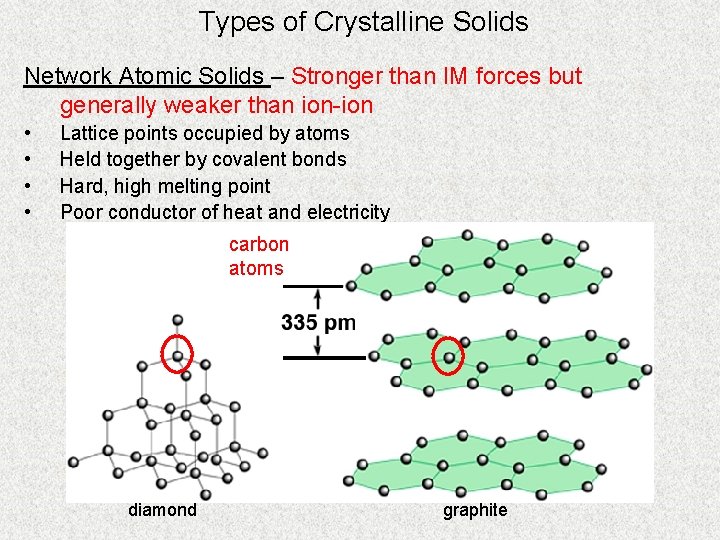
Types of Crystalline Solids Network Atomic Solids – Stronger than IM forces but generally weaker than ion-ion • • Lattice points occupied by atoms Held together by covalent bonds Hard, high melting point Poor conductor of heat and electricity carbon atoms diamond graphite

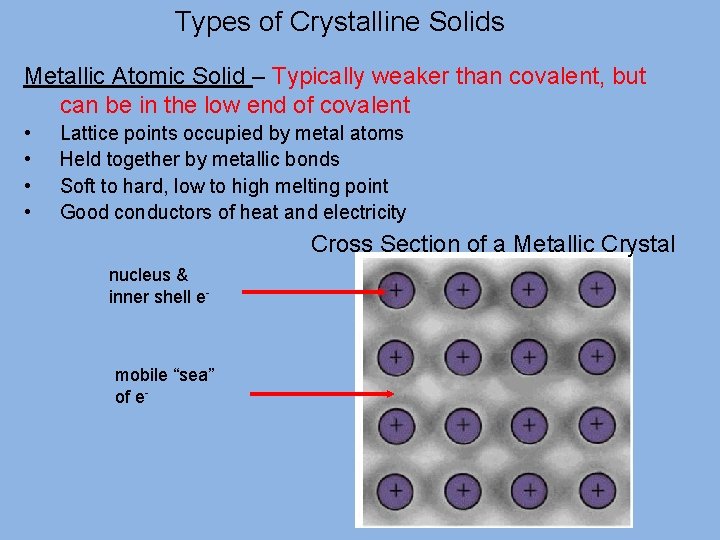
Types of Crystalline Solids Metallic Atomic Solid – Typically weaker than covalent, but can be in the low end of covalent • • Lattice points occupied by metal atoms Held together by metallic bonds Soft to hard, low to high melting point Good conductors of heat and electricity Cross Section of a Metallic Crystal nucleus & inner shell e- mobile “sea” of e-

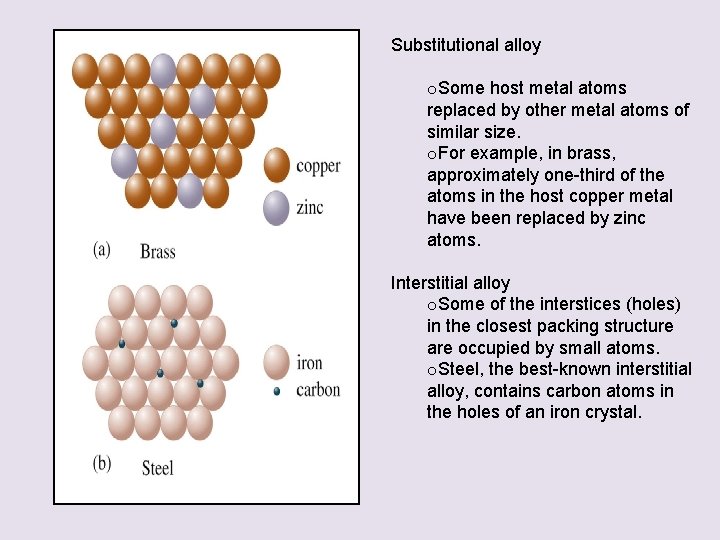
Metal Alloys • An alloy is defined as a substance that contains a mixture of elements and has metallic properties • Classified into two types: Substitutional alloy Interstitial alloy

Substitutional alloy o. Some host metal atoms replaced by other metal atoms of similar size. o. For example, in brass, approximately one-third of the atoms in the host copper metal have been replaced by zinc atoms. Interstitial alloy o. Some of the interstices (holes) in the closest packing structure are occupied by small atoms. o. Steel, the best-known interstitial alloy, contains carbon atoms in the holes of an iron crystal.

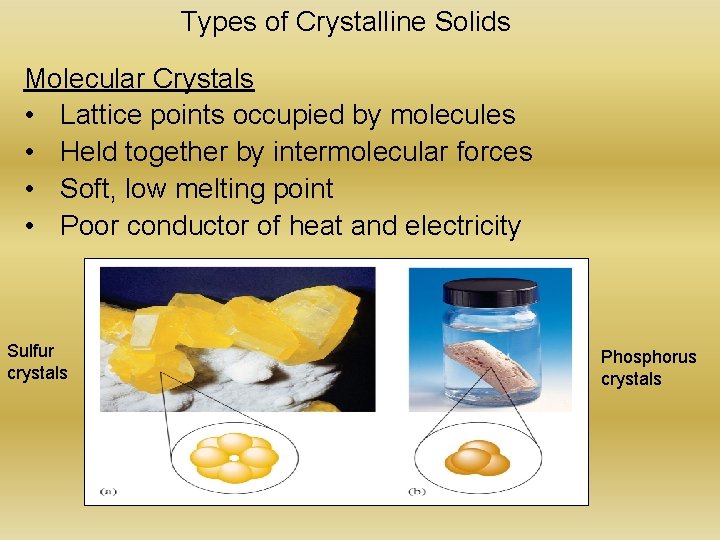
Types of Crystalline Solids Molecular Crystals • Lattice points occupied by molecules • Held together by intermolecular forces • Soft, low melting point • Poor conductor of heat and electricity Sulfur crystals Phosphorus crystals
 High surface tension vs low surface tension
High surface tension vs low surface tension Contoh tegangan antarmuka
Contoh tegangan antarmuka Solid object relationship model
Solid object relationship model Joints in carpentry
Joints in carpentry State the properties of liquid state
State the properties of liquid state Buoyancyability
Buoyancyability Liquid to gas
Liquid to gas Properties of a solid
Properties of a solid Liquid information
Liquid information Constant rate filtration equation
Constant rate filtration equation What is a force that opposes motion through direct contact
What is a force that opposes motion through direct contact Whats capillary action
Whats capillary action Surface tension biology
Surface tension biology