Pro Max Tutorial Terry A Ring CH EN




- Slides: 9

Pro. Max Tutorial Terry A. Ring CH EN 4253

Process Simulation Software • Steady State Process Simulation – – – Aspen. Plus Pro. Max Chem. Cad Hysis Hy. Sim Pro. Sim CADSim OLI Process Simulator Kem. Simp Chemical Workbench Code Ascend IV • Dynamic Process Simulation – Aspen Dynamics – CADSim – Simulation Solutions, Inc.

Overview Differences to Aspen • Aspen – Next Button – Many units that perform a given function • Degrees of Freedom are chosen for you – Setup for kinetic reactions are tricky • Pro. Max – More Thermo Package Choices – Fewer units that perform a given function • You choose the Degrees of Freedom – Reaction setup is more straight forward – Detailed design of • HX – Reboilers – Condensers – Accounts for particle sizes • Simple block models – Automatic Plant Costing (Icarus) • Distillation Columns – Does not deal with particle sizes – No Plant Costing – Some minor bugs not worked out

Steps to Run • Aspen (Left Hand Bar) – – – Title Components Thermopackage Process Flow Sheet Feed Stream Unit Specifications • Fixed degrees of freedom – Run – Results – Report • Pro. Max – Environment • Components • Thermopackage – Process Flow Sheet – Feed Stream – Unit Specifications • Various degrees of freedom – Run – Results – Report

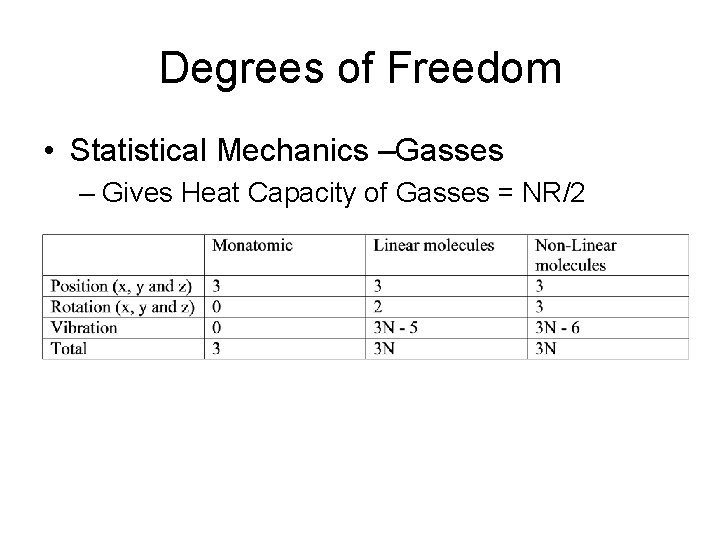
Degrees of Freedom • Statistical Mechanics –Gasses – Gives Heat Capacity of Gasses = NR/2

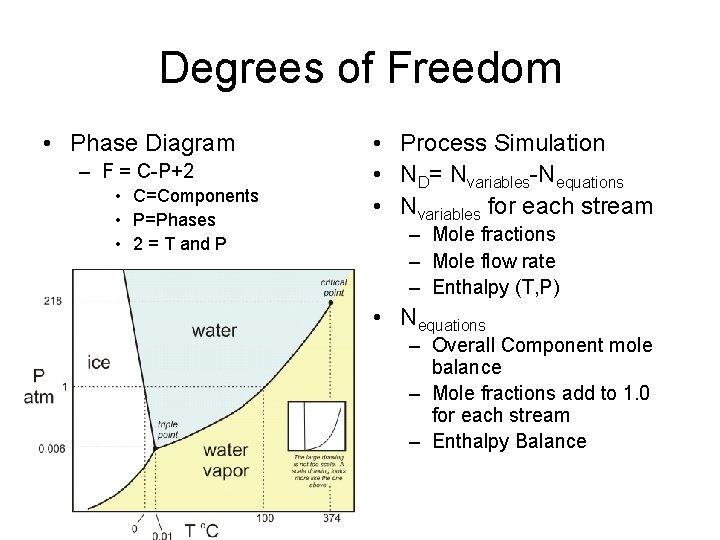
Degrees of Freedom • Phase Diagram – F = C-P+2 • C=Components • P=Phases • 2 = T and P • Process Simulation • ND= Nvariables-Nequations • Nvariables for each stream – Mole fractions – Mole flow rate – Enthalpy (T, P) • Nequations – Overall Component mole balance – Mole fractions add to 1. 0 for each stream – Enthalpy Balance

Example-I • Problem 5. 12 • Alternatives in preparing a feed. A process under design requires that 100 lbmol/hr of toluene at 70 F and 20 psia be brought to 450 F and 75 psia. • Flow sheets using Peng-Robinson – Boil-Superheat-Compress – Pump to 75 psi-Boil-Superheat

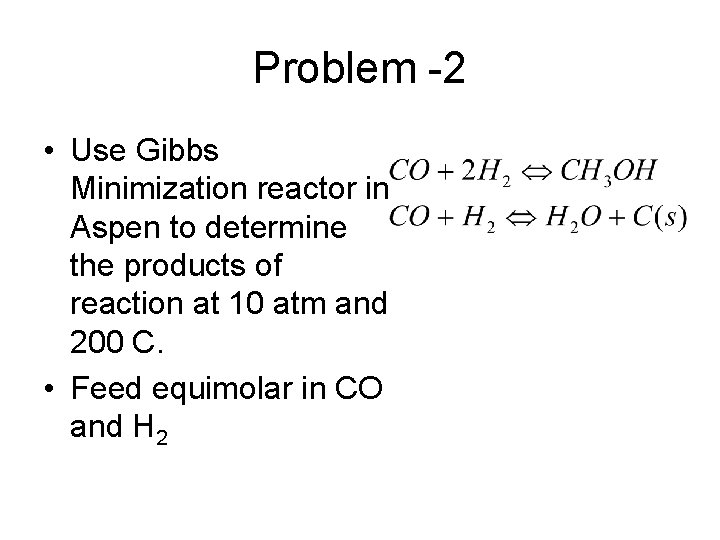
Problem -2 • Use Gibbs Minimization reactor in Aspen to determine the products of reaction at 10 atm and 200 C. • Feed equimolar in CO and H 2

Example-4 • Distillation/Flash • Isopropanol – Water – 100 lbmole/hr • Flash at 90 C • Distillation – R=2 – BUR=3