Preventing Unintended Pregnancy Prescription and Management of Contraception








































- Slides: 70

Preventing Unintended Pregnancy: Prescription and Management of Contraception Amy Whitaker MD MS Assistant Professor Department of Obstetrics and Gynecology Section of Family Planning and Contraceptive Research The University of Chicago ACHA Annual Clinical Meeting, May 2012

Financial Disclosures • I have NO actual or potential conflict of interest in relation to this educational activity or presentation. • I have no affiliation with any of the companies whose products are mentioned in this presentation.

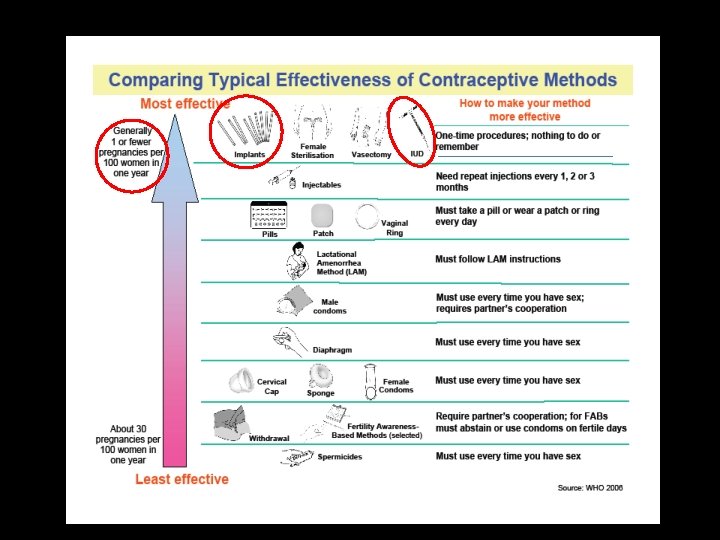
Learning Objectives At the end of this session, you should be able to: • Discuss contraceptive methods with patients, from most to least effective. • Evaluate patients to help them select a contraceptive method that will maximize correct use and continuation. • Manage side effects of contraception. • Identify appropriate patients for use of long-acting reversible contraception (LARC).

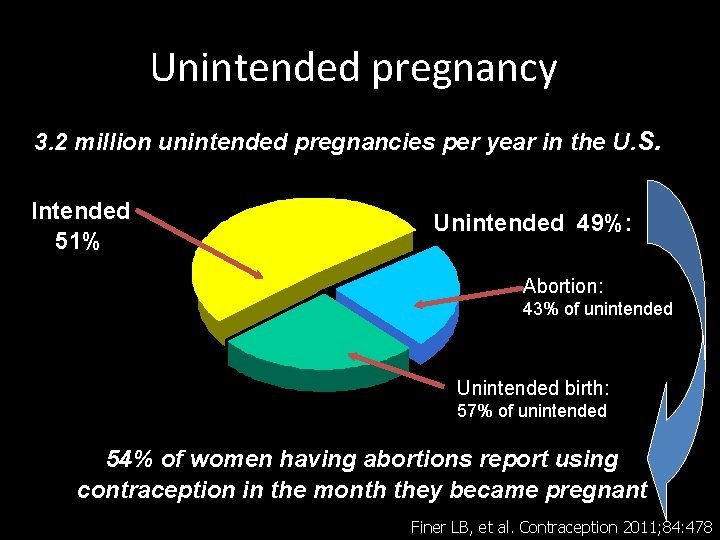
Unintended pregnancy 3. 2 million unintended pregnancies per year in the U. S. Intended 51% Unintended 49%: Abortion: 43% of unintended Unintended birth: 57% of unintended 54% of women having abortions report using contraception in the month they became pregnant Finer LB, et al. Contraception 2011; 84: 478

Unintended Pregnancy • Sexually active young adult women are at high risk for unintended pregnancy • Highest rate among young adult women & older teens – 107 / 1, 000 women age 20 -24 / year – 103 / 1, 000 women age 18 -19 / year • That’s 10% per year! Finer LB, et al. Contraception 2011; 84: 478

CDC Medical Eligibility Criteria Systematic review of evidence – Released 2010 Category Definition 1 à No restriction for use of the method 2 à Advantages of using the method generally outweigh theoretical or proven risks 3 à Theoretical or proven risks usually outweigh the advantages of using the method 4 à An unacceptable health risk if the contraceptive method is used

Long-acting Reversible Contraception LARC


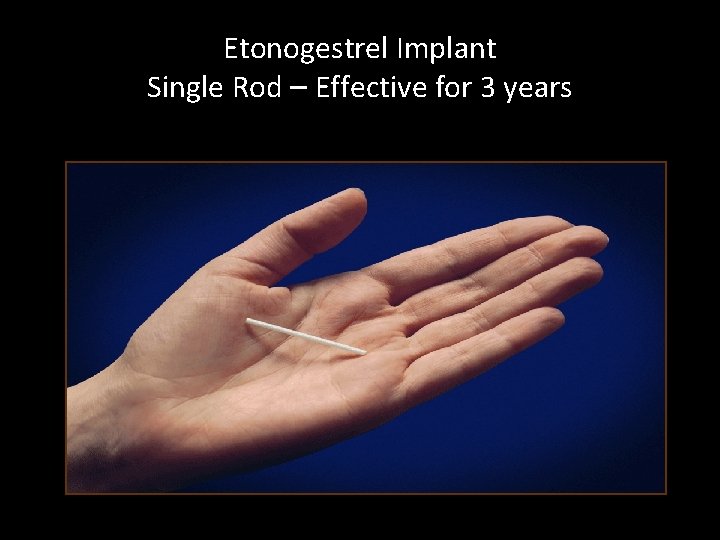
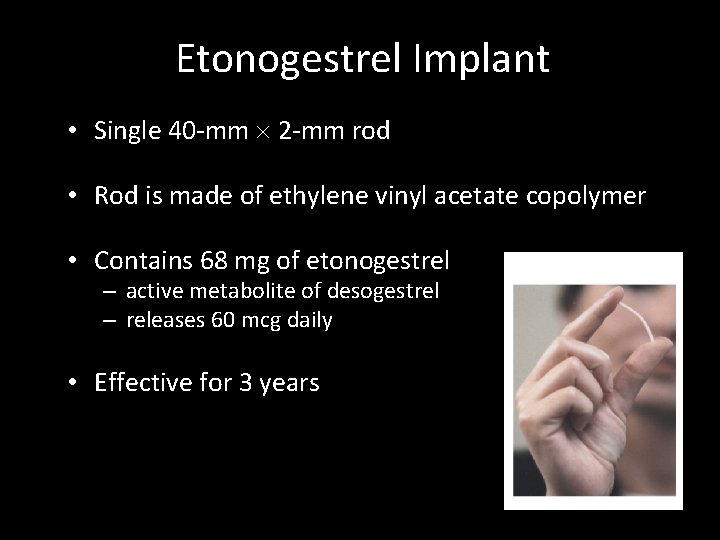
Etonogestrel Implant Single Rod – Effective for 3 years

Etonogestrel Implant • Single 40 -mm 2 -mm rod • Rod is made of ethylene vinyl acetate copolymer • Contains 68 mg of etonogestrel – active metabolite of desogestrel – releases 60 mcg daily • Effective for 3 years

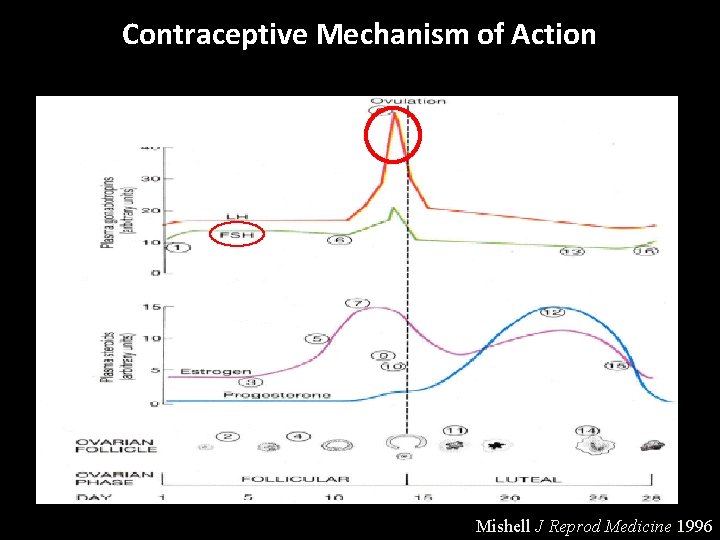
Contraceptive Mechanism of Action Mishell J Reprod Medicine 1996

Etonogestrel Implant Efficacy • More effective than permanent sterilization – 0. 05% typical (and perfect-use) failure • No pregnancies during 1200 woman-years of exposure (Pearl Index, 0; 95% CI 0. 0 -0. 2) • American study of 330 women aged 18 -40 – no pregnancies in 2 years Croxatto HB. Eur J Contracept Reprod Health Care. 2000; 5(suppl 2): 21 Funk et al. Contraception 2005; 71: 319 Trussell. Contraception 2011; 83: 397

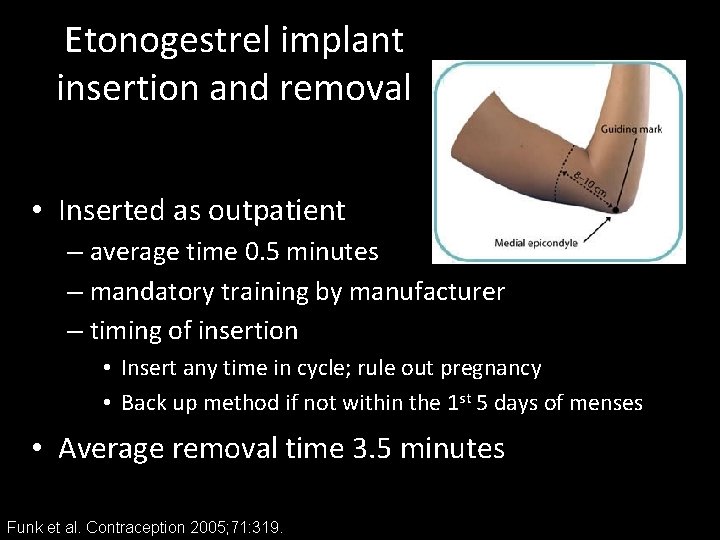
Etonogestrel implant insertion and removal • Inserted as outpatient – average time 0. 5 minutes – mandatory training by manufacturer – timing of insertion • Insert any time in cycle; rule out pregnancy • Back up method if not within the 1 st 5 days of menses • Average removal time 3. 5 minutes Funk et al. Contraception 2005; 71: 319.

Implanon® and Nexplanon®

Etonogestrel Implant Bleeding Patterns • Total number of bleeding/spotting days decreased or similar for majority of users • Key difference: – irregularity and unpredictability • ~20% amenorrhea in 1 st year – Increases to 30 -40% after 1 st year Mansour et al. Eur J Contr Reprod Health Care 2008; 13 S 1: 13

Etonogestrel implant bleeding patterns Funk et al. Contraception 2005; 71: 319.

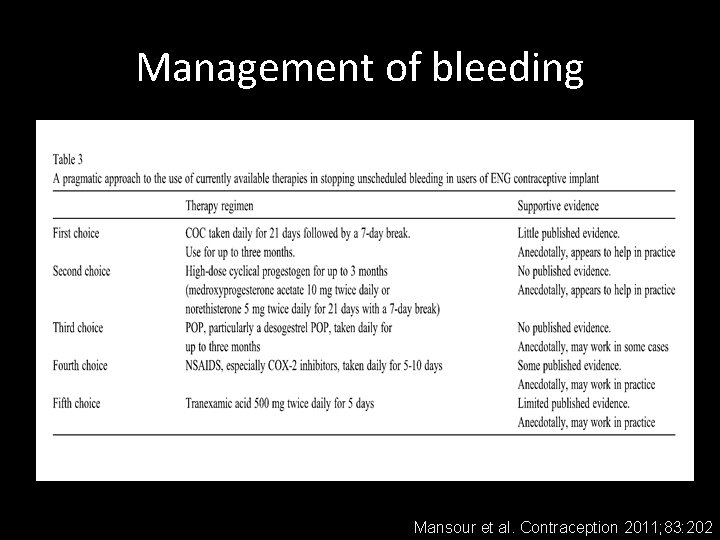
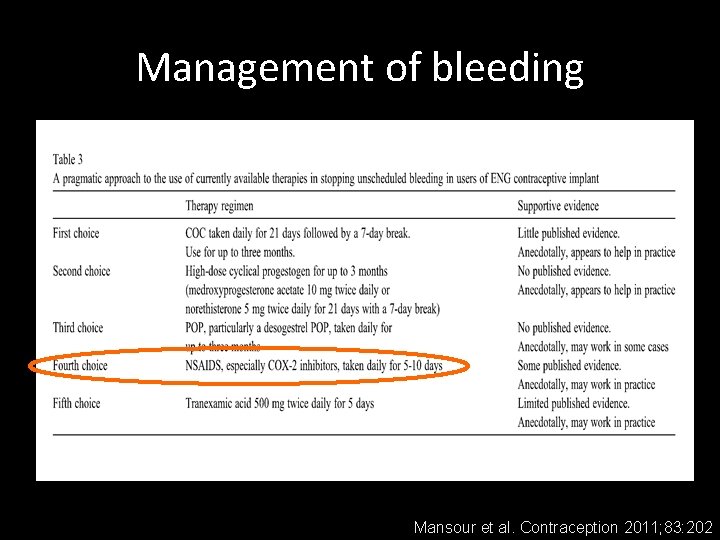
Management of bleeding Mansour et al. Contraception 2011; 83: 202

Management of bleeding 1 st Choice Daily COC for 21 days, followed by 7 -day break. Use for up to 3 months. 2 nd Choice High-dose progestin for 21 days with 7 -day break (e. g. medroxyprogesterone acetate 10 mg twice daily). Use for up to 3 months. Mansour et al. Contraception 2011; 83: 202

Management of bleeding Mansour et al. Contraception 2011; 83: 202

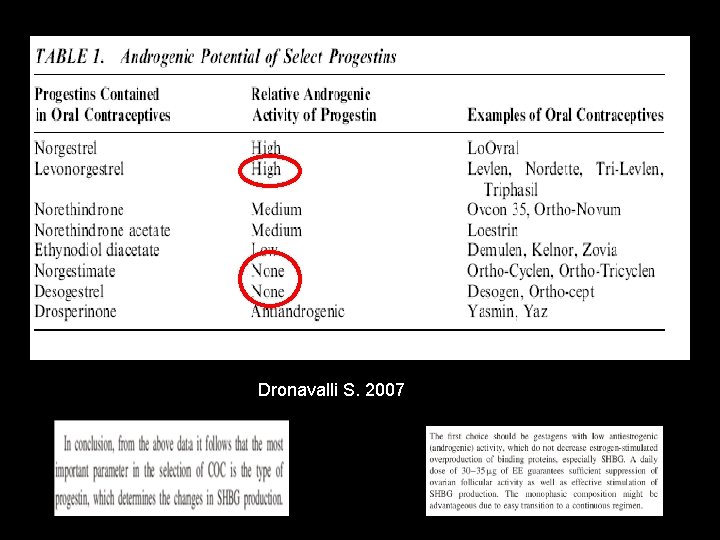
Dronavalli S. 2007

Other Side Effects • Acne – 17% reported acne – 1. 3% of women discontinued for acne – 61% of women with acne at baseline reported improvement, and only 8% worsened Funk et al. Contraception 2005; 71: 319.

Other Side Effects • Weight gain – Overall increase in BMI 0. 7 kg/m 2 • Not a significant increase – 12. 7% of women reported weight gain – 3. 3% of women discontinued for weight gain Funk et al. Contraception 2005; 71: 319.

Efficacy in overweight women • No clinical trial data • Women >130% ideal body weight excluded • Only total of 134 women > 70 kg: no failures • Small pharmacokinetics study indicates projection of hormone levels sufficient to inhibit ovulation • Not contraindicated in obese women or girls Gilliam et al. Contraception. 2011; published abstract Edelman A. SFP Guidelines. Contraception 2009; 80: 583.

Bone Mineral Density & Etonogestrel Implant • Implanon does not suppress estrogen levels to extent that Depo-Provera® does • Randomized trial of Implanon and copper IUD – No differences in BMD changes between the two groups during one year of use Beerthuizen et al. Human Reproduction 2000; 15: 118

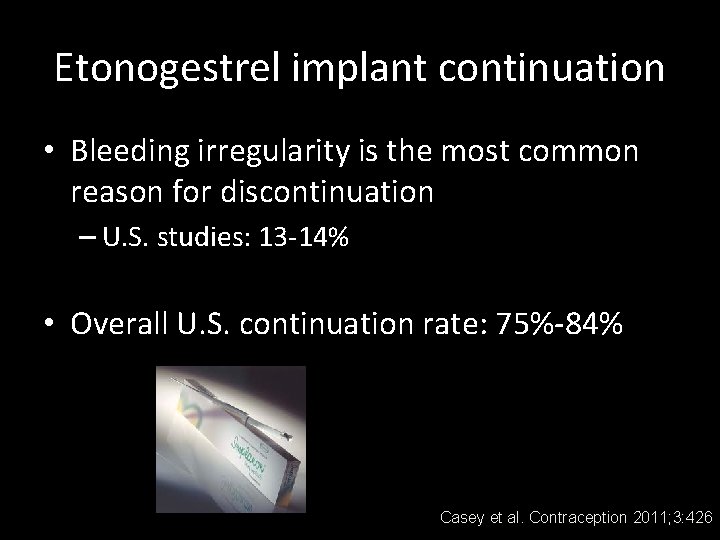
Etonogestrel implant continuation • Bleeding irregularity is the most common reason for discontinuation – U. S. studies: 13 -14% • Overall U. S. continuation rate: 75%-84% Casey et al. Contraception 2011; 3: 426

Very few contraindications • SLE with anti-phospholipid antibodies • Hepatocellular adenoma • Discontinue if develops during use: – Migraines with aura • Unexplained vaginal bleeding suspicious for serious condition, before evaluation

Appropriate patients • Women desiring highly effective, confidential, “forgettable” contraception • Women who cannot use estrogen • Tolerant of irregular bleeding – The importance of counseling

Intrauterine Contraception Copper T 380 A (Paragard®) Levonorgestrel-Releasing Intrauterine System (Mirena®)

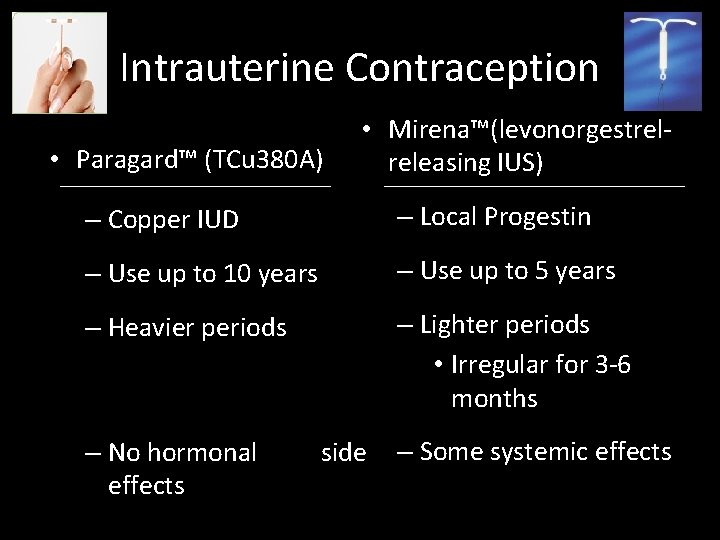
Intrauterine Contraception • Paragard™ (TCu 380 A) • Mirena™(levonorgestrelreleasing IUS) – Copper IUD – Local Progestin – Use up to 10 years – Use up to 5 years – Heavier periods – Lighter periods • Irregular for 3 -6 months – No hormonal effects side – Some systemic effects

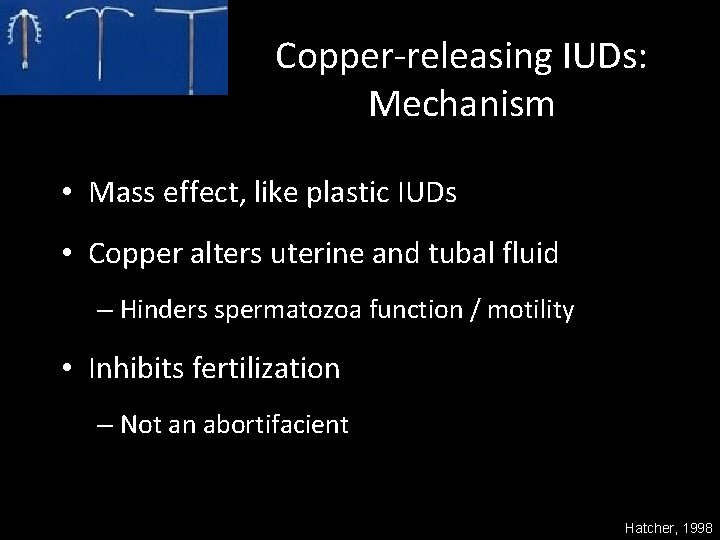
Copper-releasing IUDs: Mechanism • Mass effect, like plastic IUDs • Copper alters uterine and tubal fluid – Hinders spermatozoa function / motility • Inhibits fertilization – Not an abortifacient Hatcher, 1998

Progestin-releasing IUDs: Mechanism • Impairs spermatozoa motility / function • Inhibits conception – Unable to recover fertilized ova – Not an abortifacient • Thickens cervical mucus • Atrophy of endometrium • Impairs tubal motility • 85% of women are ovulatory Lahteenmaki, 2000

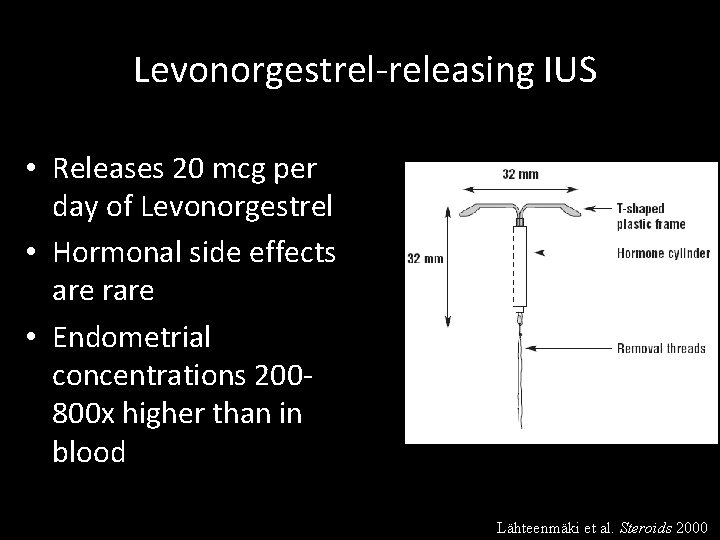
Levonorgestrel-releasing IUS • Releases 20 mcg per day of Levonorgestrel • Hormonal side effects are rare • Endometrial concentrations 200800 x higher than in blood Lähteenmäki et al. Steroids 2000

Increasing IUD use among young women • 2008: current IUD use – 3. 6% of contraceptors aged 15 -19 years • from 0% in 2002 – 5. 9% of 20 -24 year olds • from 1. 8% in 2002 • ACOG Committee Opinion December 2007 – IUDs should be offered as a “first-line choice” for contraception in both nulliparous and parous adolescents Mosher et al. National Center for Health Statistics. Vital Health Stat 2010; 23(29)

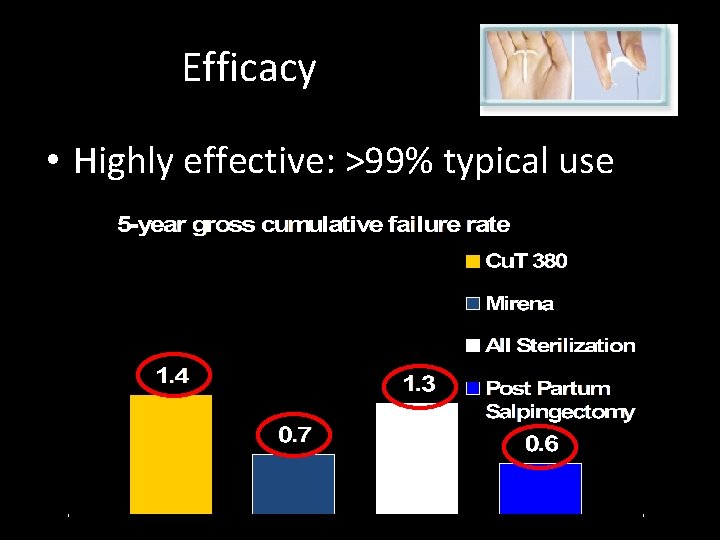
Efficacy • Highly effective: >99% typical use

Risks – younger patients • Insertion may be more difficult in nulliparous women • Higher expulsion in adolescents • May have higher rates of copper IUD removals due to bleeding and pain – No evidence this occurs with the LNG-IUS Deans et al. Contraception 2009 Behringer et al. Contraception 2011; 84: e 5 Hubacher D. Contraception 2007; 75: S 8

Copper IUDs – menstrual changes • Increased menstrual flow – Increased amount and duration – Usually no change in hemoglobin • Increased dysmenorrhea • Management – Patience and reassurance – NSAIDS around clock, start 1 day before menses

Progestin IUDs – side effects • Hormonal side effects are rare & not more common than in general population • Nonetheless, they are reasons given for discontinuation • No weight gain

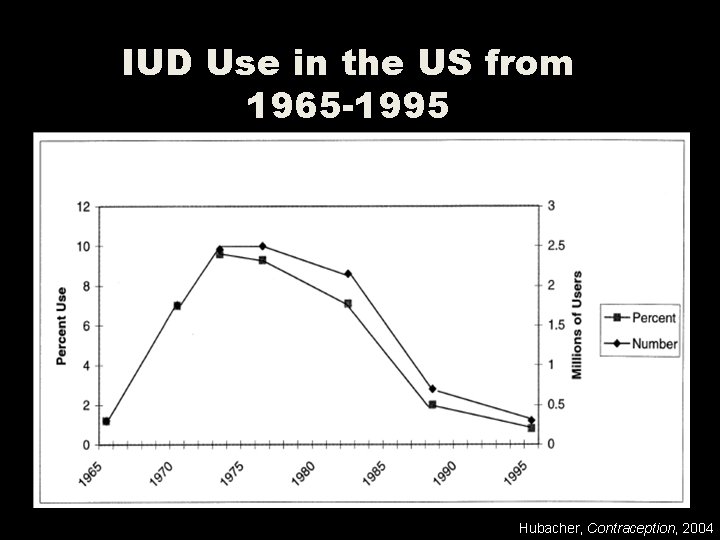
IUD Use in the US from 1965 -1995 Hubacher, Contraception, 2004

IUD and Pelvic Inflammatory Disease Risk Evidence-Based? • Re-analysis excluding the Dalkon Shield and addressing bias = no increased risk • 3 types of bias in observational studies: – Inappropriate comparison groups – Over diagnosis of PID among IUD users – Inability to control for confounding factors • WHO analysis of 22, 900 women over 8 yrs – Increased risk in first 20 days after insertion – No increased risk with continued use Grimes D. Lancet 2000; 356: 1013 Farley TM, Lancet 1992; 339: 785

What about infertility in nulliparous women? § Case-control study: 1895 women § Compared women with tubal infertility to 2 separate control groups § Non-tubal infertility (infertile controls) § Primigravid (pregnant controls) § NO association with past use of Copper IUD § Adjusted OR 1. 0 and 0. 9, respectively Hubacher et al. NEJM 2001

IUD candidates • Prior STI or PID: NOT a contraindication – Contraindicated: current PID or within the past 3 months / Current cervicitis • High risk women: screen for infection with GC or CT prior to insertion • Adolescence and nulliparity are not contraindications – CDC category 2 for age < 20 years & nulliparity CDC: U. S. Medical Eligibility Criteria for Contraceptive Use. May 2010.

Depot Medroxyprogesterone Acetate

Depo-Provera • 150 mg of depot medroxyprogesterone acetate; administered deep intramuscular or • 104 mg subcutaneous Q 3 months • Initiate anytime in cycle; rule out pregnancy – Back up method for 7 days if injection is not within 5 days of start of menses

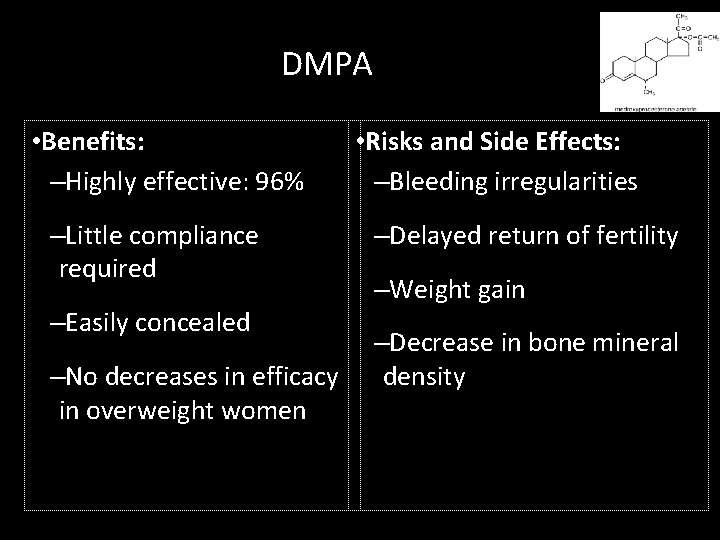
DMPA • Benefits: –Highly effective: 96% –Little compliance required –Easily concealed –No decreases in efficacy in overweight women • Risks and Side Effects: –Bleeding irregularities –Delayed return of fertility –Weight gain –Decrease in bone mineral density

DMPA – bleeding patterns • Irregular bleeding – 70% in the first year; 10% thereafter – Usually light; hemoglobin levels rise • Most common reason for discontinuation – Up to 25% in the first year of use • Management – Similar to management with Implanon

DMPA – bleeding patterns • Bleeding and spotting decrease progressively with each reinjection • Amenorrhea: – 55% at one year – 70% at 2 years – 80% at 5 years

DMPA and Weight Gain • Adult women: 4. 3 kg increase over 5 years – Compared to 1. 8 kg increase in copper IUD users • Early weight gain may predict excessive gain • Weight gain greater in adolescents who are overweight (BMI >30) when initiating DMPA – 20 lb weight gain over 18 months in obese teens Bahamondes et al. Contraception 2001; 64: 223. Le et al. Obstet Gynecol 2009; 114: 279. Bonny et al. Arch Pediatr Adolesc Med 2006; 160: 40.

FDA Black Box Warning Women who use Depo-Provera Contraceptive Injection may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible. It is unknown if use of Depo-Provera Contraceptive Injection during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk of osteoporotic fracture in later life. Depo-Provera Contraceptive Injection should be used as a long-term birth control method (eg, longer than 2 years) only if other birth control methods are inadequate (see WARNINGS).

DMPA & Bone Mineral Density (BMD) • Use of DMPA is associated with loss of BMD • After stopping, recovery of BMD is seen – return to baseline in 1 -4 years • No data on fracture risk in women who have used DMPA in the past Berenson et al. Obstet Gynecol 2004; 103 Scholes et al. Arch Pediatr Adolesc Med 2005; 159: 139 Harel et al. Contraception 2010; 81: 281

DMPA and BMD • Position papers: – WHO 1 – ACOG 2 – Society of Adolescent Medicine 3 • NONE recommend restricted initiation or continuation • NONE recommend routine BMD testing 1 WHO Epidemiological Record No. 35, 2005 2 ACOG Practice Bulletin No. 73, 2006 3 Cromer BA et al. J Adolesc Health 2006; 39: 296

Depo-Provera Few contraindications • Similar to progestin implant • Severe hypertension (>160/>100) • Diabetes with – vascular disease and / or – > 20 years disease

Appropriate patients • Women desiring effective, confidential, method and who can return for injections • Women who cannot use estrogen • Tolerant of irregular bleeding • Special populations – Sickle cell disease – Epilepsy

Combined Hormonal Contraceptives

Combined Hormonal Contraceptives • Safe for most young women • Added benefit of regulation of menses • “Typical-use” effectiveness ~ 92% – (Do not quote perfect use ~ 99%)

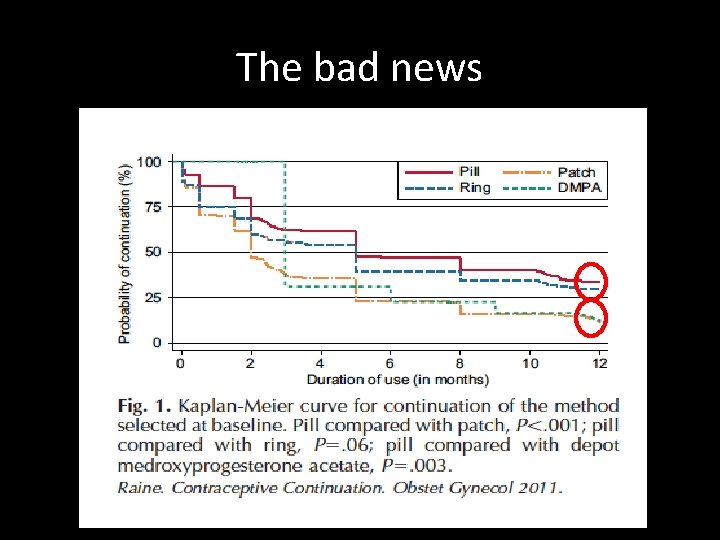
Oral Contraceptive Pills • Commonly reported as most effective method – 54% of 15 -19 year olds who use contraception – 48% of 20 -24 year olds • Poor continuation – Up to 50% discontinue within first 3 cycles – One study found discontinuation 88% at one year – College students: only 29% still using at 6 months Mosher et al. National Center for Health Statistics. Vital Health Stat 2010; 23(29) Rosenberg MJ, et al. Contraception 1995; 52: 137 Zibners A, et al. J Pediatr Adolesc Gynecol 1999; 12: 90

Contraceptive patch • Weekly transdermal patch – 20 mcg EE + 150 mcg norelgestromin daily • Continuous delivery – Area “under the curve” is 60% > in a 35 mcg pill – Possible increase in estrogen side effects such as VTE • Less effective if body weight > 90 kg Contraception 2005 Sep; 72(3): 168 -74 Abma et al. National Center for Health Statistics. Vital Health Stat 2010; 23(30)

The vaginal ring • 3 weeks with 1 week ring-free interval – or 24 -4, or continuous • Lowest ethinyl estradiol dose (15 mcg EE, 120 mcg etonorgestrel daily) • Continuous dosing • Use back-up method if out for > 3 hours Abma et al. National Center for Health Statistics. Vital Health Stat 2010; 23(30 )

Pharmacokinetics of patch, ring and OCPs

Potential advantages of new delivery systems • Compliance – Patch > pill in some studies…but not in others • Satisfaction – No consistent differences • Side effects – Ring with some improvement in nausea and cycle control • Continuation and efficacy O’Connell et al. Clin Obstet Gynecol 2007; 50: 918 Brache et al. Contraception 2010; 82: 418.

The bad news

Weight and Combined Hormonal Contraceptives CHC and Weight gain – NO LINK Gallo et al. Cochrane Dbase of Systematic Reviews 2008, Issue 4. Art. No. : CD 003987. DOI: 10. 1002/14651858. CD 003987. pub 3.

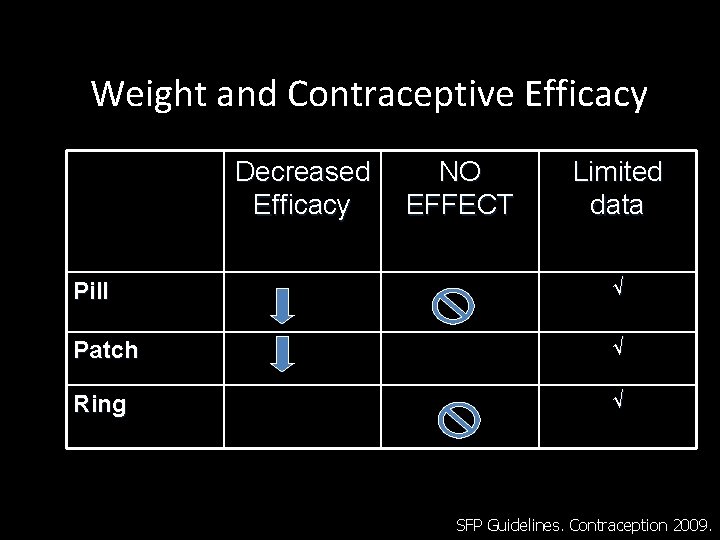
Weight and Contraceptive Efficacy Decreased Efficacy NO EFFECT Limited data Pill √ Patch √ Ring √ SFP Guidelines. Contraception 2009.

Alternate regimens • Many options beyond standard 21 -7 – 24 -4 (e. g. Yaz, Loestrin® 24 Fe) – 3 -month [84 -7] (e. g. Seasonale, Seasonique) – 12 -months (e. g. Lybrel) • Advantages – Decreased follicular activity • Potential for increased efficacy – Fewer bleeding days • More breakthrough bleeding in first several months

Breakthrough bleeding • Common reason for discontinuation • With time, improved with extended regimens • Higher rates in women – who smoke – with cervical infections • Management: – If near end of cycle, discontinue early – If severe, consider exogenous estrogen

Acne • All pills studied reduced acne compared to placebo • No consistent results regarding different types of progestins – Cyproterone (pregnane) may be better than LNG – LNG may be better than desogestrel (!) Arowojoluet al. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No. : CD 004425. DOI: 10. 1002/14651858. CD 004425. pub 4

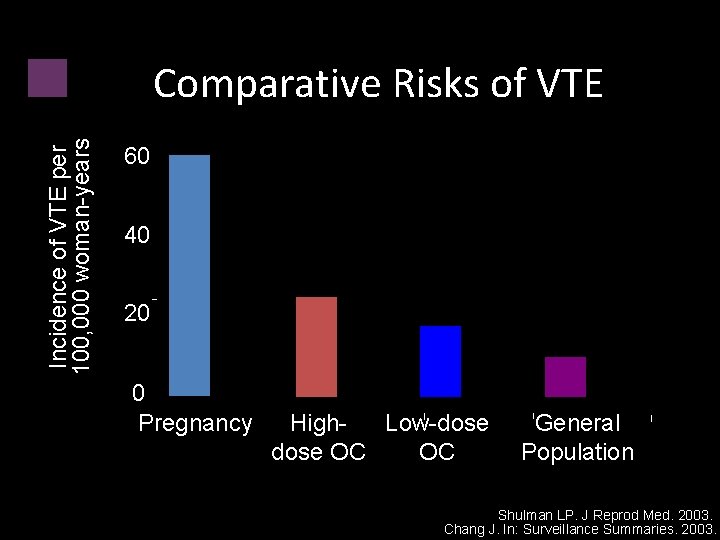
Incidence of VTE per 100, 000 woman-years Comparative Risks of VTE 60 40 20 0 Pregnancy High. Low-dose OC OC General Population Shulman LP. J Reprod Med. 2003. Chang J. In: Surveillance Summaries. 2003.

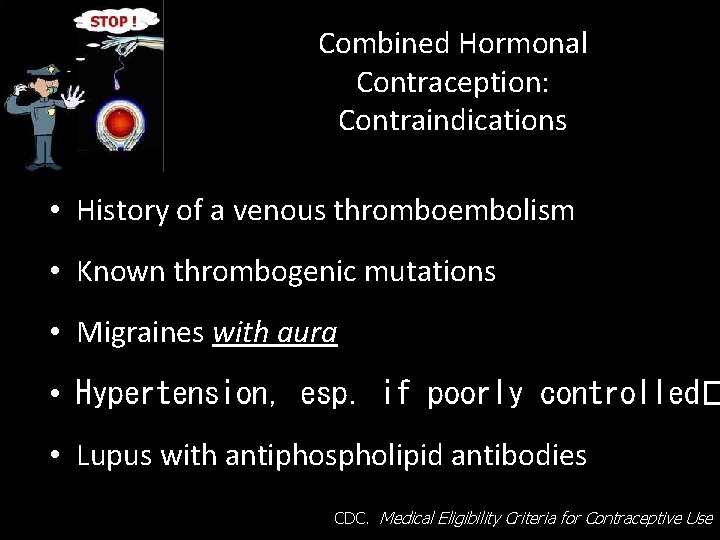
Combined Hormonal Contraception: Contraindications • History of a venous thromboembolism • Known thrombogenic mutations • Migraines with aura • Hypertension, esp. if poorly controlled� • Lupus with antiphospholipid antibodies CDC. Medical Eligibility Criteria for Contraceptive Use

Barrier Methods

Condoms • Safe for all young women • Only method that offers – PROTECTION FROM STIs • Not effective enough for contraception – Typical-use failure is 15% • Counsel for dual protection from pregnancy & STIs

Summary • Unplanned pregnancy is common • The implant and IUDs provide greater: – Convenience – Efficacy • Discontinuation of all methods is high • Counseling in advance is vital
 Pictures of spotting during pregnancy
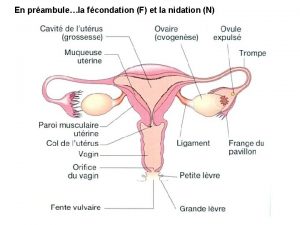
Pictures of spotting during pregnancy Ogn and pgn full form in contraception
Ogn and pgn full form in contraception Contraception sterilisation and abortion act 1977
Contraception sterilisation and abortion act 1977 Victorian era birth control
Victorian era birth control Diaporama sur la contraception
Diaporama sur la contraception Contraception for over 40s
Contraception for over 40s Contraception
Contraception Diaphragme contraceptif
Diaphragme contraceptif Voluntary surgical contraception
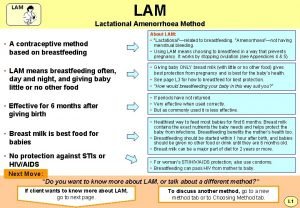
Voluntary surgical contraception What is lam contraception
What is lam contraception Mode d'action de la pilule contraceptive
Mode d'action de la pilule contraceptive Unintended consequences
Unintended consequences Unintended consequences
Unintended consequences Chapter 24 lesson 2 preventing and treating stds
Chapter 24 lesson 2 preventing and treating stds Chapter 9 lesson 2 resolving conflicts
Chapter 9 lesson 2 resolving conflicts Chapter 14:3 observing fire safety
Chapter 14:3 observing fire safety Chapter 9 resolving conflicts and preventing violence
Chapter 9 resolving conflicts and preventing violence Chapter 13:2 preventing accidents and injuries
Chapter 13:2 preventing accidents and injuries Chapter 20 preventing kitchen accidents
Chapter 20 preventing kitchen accidents Chapter 16 preventing infection
Chapter 16 preventing infection Preventing kitchen accidents worksheet
Preventing kitchen accidents worksheet How do the 6cs prevent discrimination
How do the 6cs prevent discrimination Chapter 4 preventing injuries through fitness
Chapter 4 preventing injuries through fitness Chapter 15 preventing infection
Chapter 15 preventing infection Preventing hand injuries
Preventing hand injuries Work comp puncture
Work comp puncture Preventing ageing unequally
Preventing ageing unequally Which is mainly responsible for preventing erosion
Which is mainly responsible for preventing erosion What is visitor pre registration in picme
What is visitor pre registration in picme Medication order sample
Medication order sample Apa yang dimaksud dengan resep makanan
Apa yang dimaksud dengan resep makanan Pregnancy and fetal development brainpop answers
Pregnancy and fetal development brainpop answers Mississippi classification of hellp syndrome
Mississippi classification of hellp syndrome Prenatal care and adaptations to pregnancy
Prenatal care and adaptations to pregnancy Lesson 20.2 the male reproductive system
Lesson 20.2 the male reproductive system Chapter 13 anatomy and physiology of pregnancy
Chapter 13 anatomy and physiology of pregnancy Trimester graphic organizer
Trimester graphic organizer Icd 10 code for twin pregnancy
Icd 10 code for twin pregnancy Adoloscense
Adoloscense Chapter 4 prenatal care and adaptations to pregnancy
Chapter 4 prenatal care and adaptations to pregnancy What is tender breast in pregnancy
What is tender breast in pregnancy Rpd lab prescription
Rpd lab prescription Medication lot number
Medication lot number Cylinder transposition
Cylinder transposition Elements of prescription
Elements of prescription Principles of prescription writing
Principles of prescription writing Easement by prescription
Easement by prescription Costco pharmacy regent
Costco pharmacy regent Signatura prescription
Signatura prescription Prescription label parts
Prescription label parts Illinois prescription monitoring
Illinois prescription monitoring Fitt prescription
Fitt prescription Elements of a prescription
Elements of a prescription Prescription terms
Prescription terms Exercise prescription example
Exercise prescription example Nvo eye prescription
Nvo eye prescription Prescription parts
Prescription parts Local 237 dental
Local 237 dental Prescription abbreviations
Prescription abbreviations Principles of prescription
Principles of prescription Prescription abbreviations
Prescription abbreviations Prescriptii
Prescriptii Peritoneal dialysis prescription calculator
Peritoneal dialysis prescription calculator How to write a veterinary prescription
How to write a veterinary prescription Individual prescription order system disadvantages
Individual prescription order system disadvantages Prescription clerk training
Prescription clerk training Total life care rx pharmacy
Total life care rx pharmacy Writing by
Writing by E rd
E rd Drug basket method
Drug basket method Transposing plus cylinder to minus
Transposing plus cylinder to minus