Potential risks of GM crops 1 Economic risks












- Slides: 29

Potential risks of GM crops 1. Economic risks: transgene silencing: losing trait 2. Environmental a. pollen mediated escape b. volunteer seed mediated dispersal c. horizontal gene transfer 3. Health: unknown allergens



Heteroencapsidation has long been recognized in mixed infections involving different groups of aphid-borne viruses that are transmitted in a persistent, semi-persistent or non-persistent manner. Exchange of capsid subunits between viruses of the same group can be responsible for the modification of vector specificity. In another case, a non-aphid transmitted (NAT) strain may become aphid-transmissible through phenotypic mixing. Heteroencapsidation has also been observed between the coat protein (CP) produced in transgenic plants and the CP of incoming homologous or heterologous viruses (Osbourne et al. , 1989; Farinelli et al. , 1992; Lecoq et al. , 1993). In the latter report, a ZYMV-NAT strain was transmitted by aphids when encapsidated with a plum pox potyvirus (PPV) CP synthesized in transgenic Nicotiana benthamiana plants.



Journal of General Virology (1998), 79, 1509– 1517 Use of modified plum pox virus coat protein genes developed to limit heteroencapsidation-associated risks Aphid transmission of a non-aphid-transmissible strain of zucchini yellow mosaic virus (ZYMV-NAT) occurs in transgenic plants expressing the plum pox potyvirus (PPV) coat protein (CP) gene. Heteroencapsidation has been shown to be responsible for this modification in the epidemiological characteristics of the infecting virus. In order to prevent this biological risk, several modified PPV CP constructs were produced that were designed to interfere with heteroencapsidation itself or to block aphid transmission of heteroencapsidated virions. These constructs were first expressed in Escherichia coli in order to check for the accumulation of pseudoparticles by electron microscopy. Virus-like particles (VLPs) were found with the full-length CP and with a PPV CP lacking the DAG amino acid triplet involved in aphid transmission. However, no VLPs were observed with CP lacking R 220, Q 221 or D 264, amino acids known to be essential for the assembly of other potyvirus CPs. Transgenic Nicotiana benthamiana lines expressing the different PPV CP constructs were infected with ZYMV-NAT. Aphid transmission assays performed with these plants demonstrated that the strategies developed here provide an effective means of minimizing the biological risks associated with heteroencapsidation.

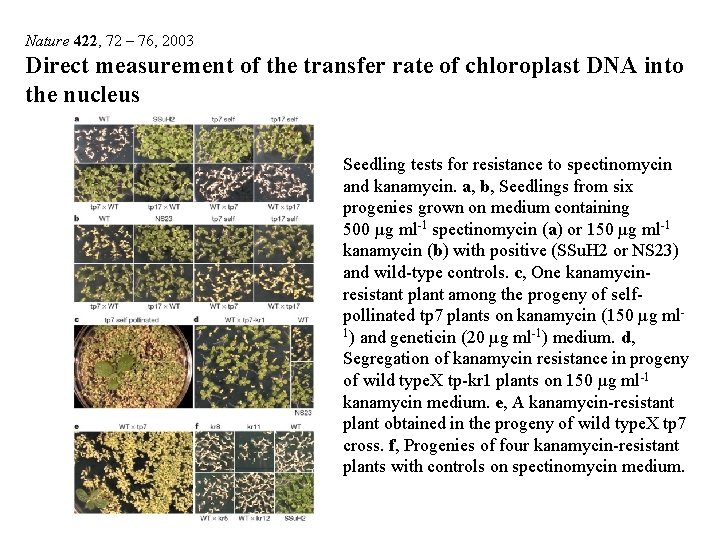
Nature 422, 72 – 76, 2003 Direct measurement of the transfer rate of chloroplast DNA into the nucleus Seedling tests for resistance to spectinomycin and kanamycin. a, b, Seedlings from six progenies grown on medium containing 500 µg ml-1 spectinomycin (a) or 150 µg ml-1 kanamycin (b) with positive (SSu. H 2 or NS 23) and wild-type controls. c, One kanamycinresistant plant among the progeny of selfpollinated tp 7 plants on kanamycin (150 µg ml 1) and geneticin (20 µg ml-1) medium. d, Segregation of kanamycin resistance in progeny of wild type. X tp-kr 1 plants on 150 µg ml-1 kanamycin medium. e, A kanamycin-resistant plant obtained in the progeny of wild type. X tp 7 cross. f, Progenies of four kanamycin-resistant plants with controls on spectinomycin medium.

Transfer of neo to the nucleus took place in a total of 16 heritable events in 250, 000 seedlings — that is, at an incidence of around 6 X 10 -5. Molecular analyses confirmed that the neo genes, together with flanking plastid DNA of variable size, had indeed been incorporated into the tobacco nuclear DNA at different genomic locations. Nonetheless, incorporation of transgenes in plastids should still be effective for containment of those genes. If a transgene is incorporated in the nucleus, each pollen grain will carry the transgenic trait. By contrast, assuming that the probability of plastid gene expression from a broken fragment is only 100 times lower than the transfer rate of plastid DNA (nucleus translocated aad. A gene did not express because it contained plastid promoter, however, broken pieces of plastid DNA could be expressed when translocated into nucleus) , only 1 in 1. 6 million pollen grains will carry the expressed plastid gene.

Mobile genome Genome sequences show extensive tracts of mitochondrial and plastid DNA that are integrated in nuclear chromosomes. Evidence indicates that an active process of DNA translocation from organelles to the nucleus has been ongoing since the origin or organelles from free-living prokaryotes. Movement of DNA from organelles to the nucleus occurs at very high rates. These rates have been measured experimentally for mitochondria in yeast and more recently for plastids using transgenic chloroplast technology in tobacco. Phylogenetic analyses and genome comparisons show that influx of organellar DNA to the nucleus had a marked quantitative impact on the gene content of eukaryotic chromosomes. Translocated genes might be expressed to provide products that are targeted to all parts of the cell; there is no magic homing device that targets the products of transferred genes back to the organelle of their origin.

Complete organelle genomes are cropping up in eukaryotic chromosomes, so why are any genes left in organelles at all? Observations from genomes and from experimental transfers favour the view that bulk DNA from lysed organelles is the vector that is responsible for gene relocation, although in some groups of eukaryotes, RNA intermediates have been suggested to act as vectors as well. The downpour of organelle DNA into eukaryotic chromosomes is an unavoidable consequence of endosymbiosis. This mechanism of natural variation is unique to eukaryotic cells and was an important force in the genesis of eukaryotic genomes. The impact of endosymbiotic gene transfer on eukaryotic chromosomes was probably greatest in the early phases of organelle origins, before the protein import machinery of mitochondria and chloroplasts had been invented.

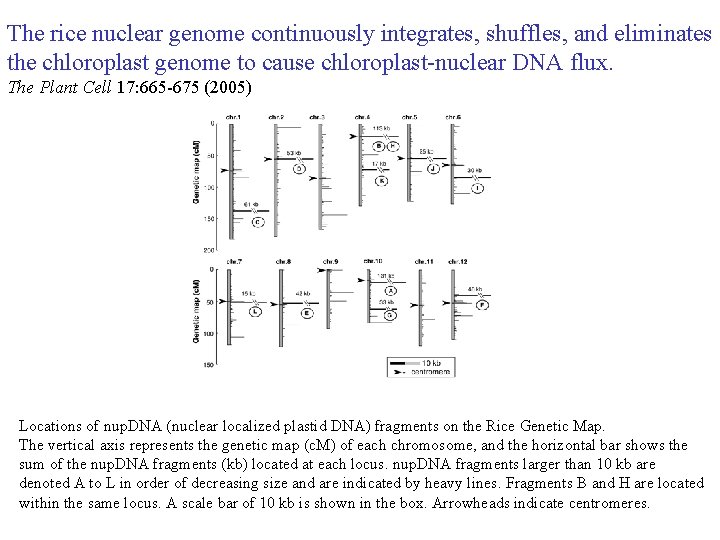
The rice nuclear genome continuously integrates, shuffles, and eliminates the chloroplast genome to cause chloroplast-nuclear DNA flux. The Plant Cell 17: 665 -675 (2005) Locations of nup. DNA (nuclear localized plastid DNA) fragments on the Rice Genetic Map. The vertical axis represents the genetic map (c. M) of each chromosome, and the horizontal bar shows the sum of the nup. DNA fragments (kb) located at each locus. nup. DNA fragments larger than 10 kb are denoted A to L in order of decreasing size and are indicated by heavy lines. Fragments B and H are located within the same locus. A scale bar of 10 kb is shown in the box. Arrowheads indicate centromeres.

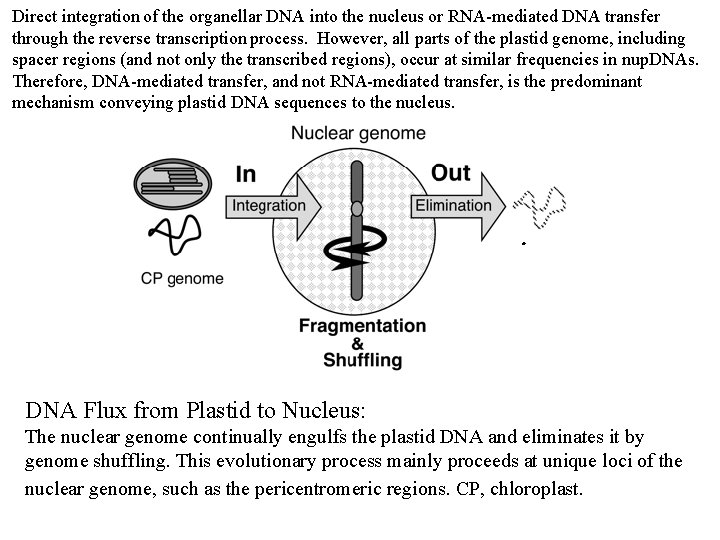
Direct integration of the organellar DNA into the nucleus or RNA-mediated DNA transfer through the reverse transcription process. However, all parts of the plastid genome, including spacer regions (and not only the transcribed regions), occur at similar frequencies in nup. DNAs. Therefore, DNA-mediated transfer, and not RNA-mediated transfer, is the predominant mechanism conveying plastid DNA sequences to the nucleus. DNA Flux from Plastid to Nucleus: The nuclear genome continually engulfs the plastid DNA and eliminates it by genome shuffling. This evolutionary process mainly proceeds at unique loci of the nuclear genome, such as the pericentromeric regions. CP, chloroplast.

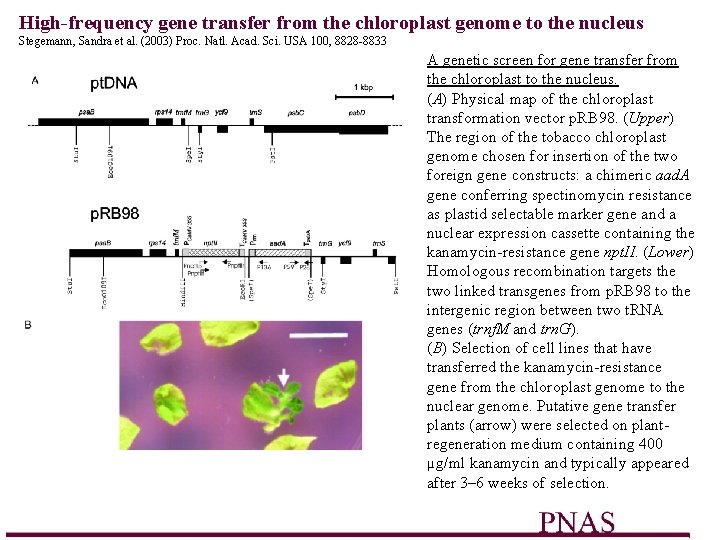
High-frequency gene transfer from the chloroplast genome to the nucleus Stegemann, Sandra et al. (2003) Proc. Natl. Acad. Sci. USA 100, 8828 -8833 A genetic screen for gene transfer from the chloroplast to the nucleus. (A) Physical map of the chloroplast transformation vector p. RB 98. (Upper) The region of the tobacco chloroplast genome chosen for insertion of the two foreign gene constructs: a chimeric aad. A gene conferring spectinomycin resistance as plastid selectable marker gene and a nuclear expression cassette containing the kanamycin-resistance gene npt. II. (Lower) Homologous recombination targets the two linked transgenes from p. RB 98 to the intergenic region between two t. RNA genes (trnf. M and trn. G). (B) Selection of cell lines that have transferred the kanamycin-resistance gene from the chloroplast genome to the nuclear genome. Putative gene transfer plants (arrow) were selected on plantregeneration medium containing 400 µg/ml kanamycin and typically appeared after 3– 6 weeks of selection.

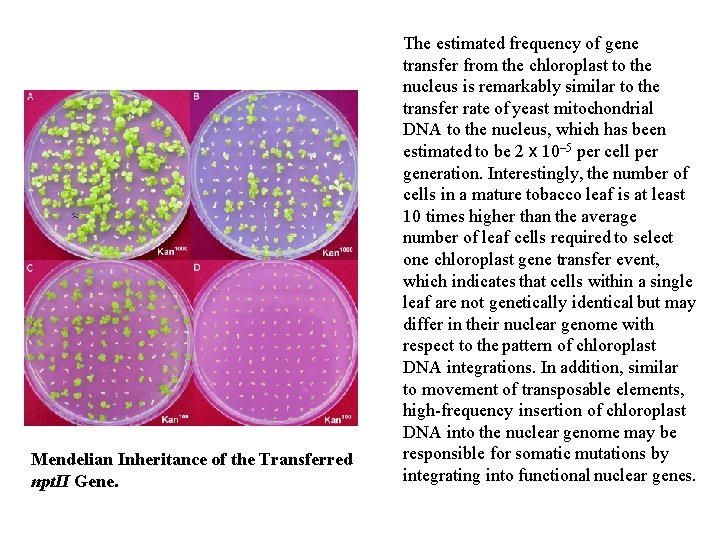
Mendelian Inheritance of the Transferred npt. II Gene. The estimated frequency of gene transfer from the chloroplast to the nucleus is remarkably similar to the transfer rate of yeast mitochondrial DNA to the nucleus, which has been estimated to be 2 x 10– 5 per cell per generation. Interestingly, the number of cells in a mature tobacco leaf is at least 10 times higher than the average number of leaf cells required to select one chloroplast gene transfer event, which indicates that cells within a single leaf are not genetically identical but may differ in their nuclear genome with respect to the pattern of chloroplast DNA integrations. In addition, similar to movement of transposable elements, high-frequency insertion of chloroplast DNA into the nuclear genome may be responsible for somatic mutations by integrating into functional nuclear genes.

Agrobacterium-mediated DNA transfer, and then some by Stanton B Gelvin In addition to its plasmid DNA, Agrobacterium tumefaciens can transfer its chromosomal DNA to plant genomes

Introgression of transgenes Invasive species can be generated by traditional breeding as well as GE. No study has conclusively examined whether introgression of transgenes has occurred into natural population. However, past experience with crop plants suggests that negative effects are possible. For 7 species (wheat, rice, soybean, sorghum, millet, beans and sunflower seeds) of the world’s top 13 crops, hybridization with the wild relatives has contributed to the evolution of weeds. In some cases, high levels of introgression from cultivated or introduced relatives have eliminated genetic diversity, effectively contributing to their extinction. The challenge is how to identify an invasive species. Simple comparisons of fecundity and survival will not adequately predict invasiveness. Variation in the competitive environment and timing of introductions can confound predictions. Unknown factors cause unexplained time lags that occur between the introduction of the species and the expansion of its population. These represent key challenge for assessing the risk of invasiveness.


In this paper authors claim sample in lane e (see below) gave strongest PCR band. Where is lane e? ? A bulk of 150 -400 grains were used to carry out PCR.



This study demonstrates that cross-pollination between commercial Canola fields occurs at low frequency but to considerable distance.


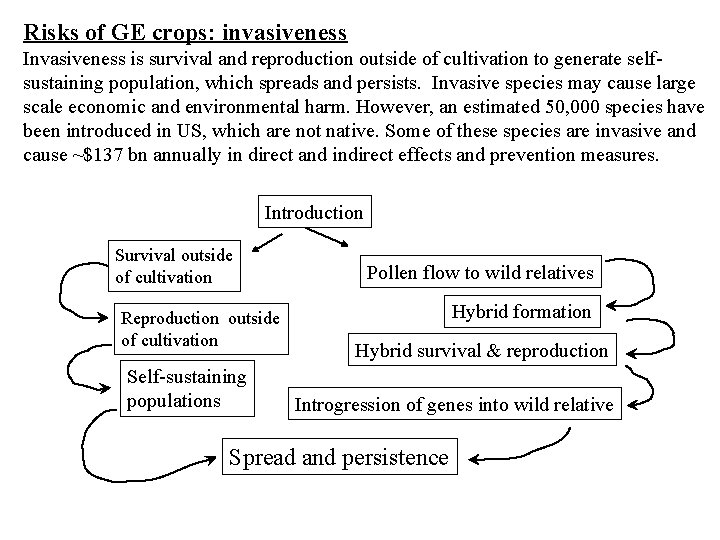
Risks of GE crops: invasiveness Invasiveness is survival and reproduction outside of cultivation to generate selfsustaining population, which spreads and persists. Invasive species may cause large scale economic and environmental harm. However, an estimated 50, 000 species have been introduced in US, which are not native. Some of these species are invasive and cause ~$137 bn annually in direct and indirect effects and prevention measures. Introduction Survival outside of cultivation Reproduction outside of cultivation Self-sustaining populations Pollen flow to wild relatives Hybrid formation Hybrid survival & reproduction Introgression of genes into wild relative Spread and persistence

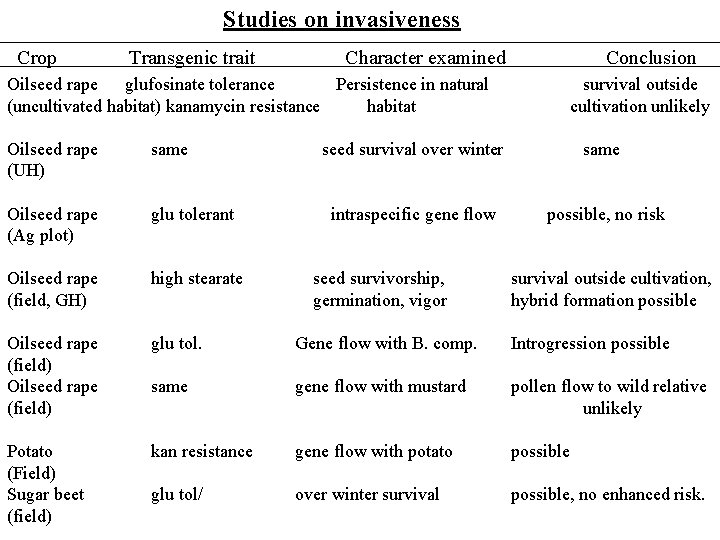
Studies on invasiveness Crop Transgenic trait Character examined Conclusion Oilseed rape glufosinate tolerance Persistence in natural (uncultivated habitat) kanamycin resistance habitat survival outside cultivation unlikely Oilseed rape (UH) same seed survival over winter same Oilseed rape (Ag plot) glu tolerant intraspecific gene flow possible, no risk Oilseed rape (field, GH) high stearate seed survivorship, germination, vigor survival outside cultivation, hybrid formation possible Oilseed rape (field) glu tol. Gene flow with B. comp. Introgression possible same gene flow with mustard pollen flow to wild relative unlikely Potato (Field) Sugar beet (field) kan resistance gene flow with potato possible glu tol/ over winter survival possible, no enhanced risk.

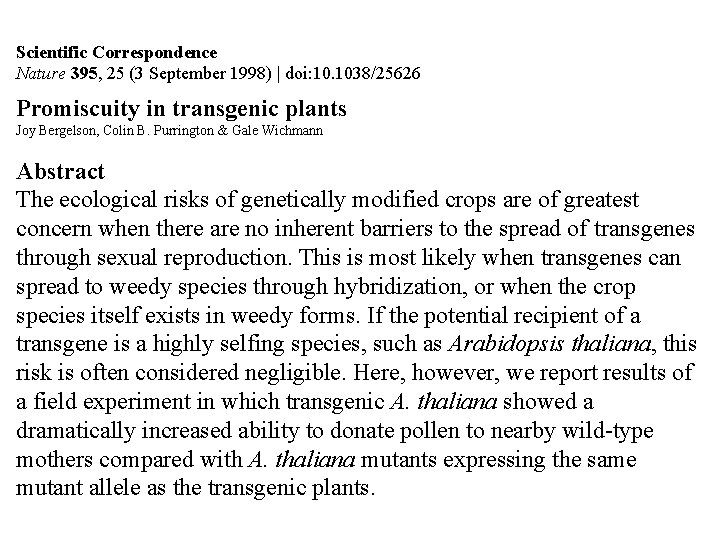
Scientific Correspondence Nature 395, 25 (3 September 1998) | doi: 10. 1038/25626 Promiscuity in transgenic plants Joy Bergelson, Colin B. Purrington & Gale Wichmann Abstract The ecological risks of genetically modified crops are of greatest concern when there are no inherent barriers to the spread of transgenes through sexual reproduction. This is most likely when transgenes can spread to weedy species through hybridization, or when the crop species itself exists in weedy forms. If the potential recipient of a transgene is a highly selfing species, such as Arabidopsis thaliana, this risk is often considered negligible. Here, however, we report results of a field experiment in which transgenic A. thaliana showed a dramatically increased ability to donate pollen to nearby wild-type mothers compared with A. thaliana mutants expressing the same mutant allele as the transgenic plants.

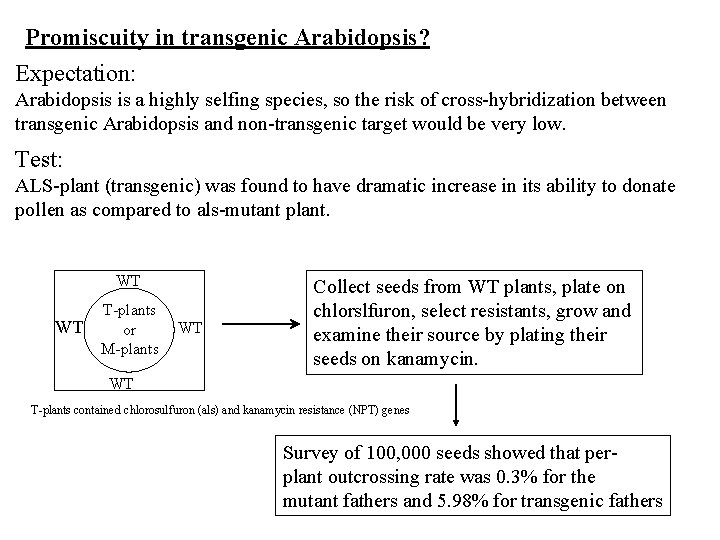
Promiscuity in transgenic Arabidopsis? Expectation: Arabidopsis is a highly selfing species, so the risk of cross-hybridization between transgenic Arabidopsis and non-transgenic target would be very low. Test: ALS-plant (transgenic) was found to have dramatic increase in its ability to donate pollen as compared to als-mutant plant. WT WT T-plants or M-plants WT Collect seeds from WT plants, plate on chlorslfuron, select resistants, grow and examine their source by plating their seeds on kanamycin. WT T-plants contained chlorosulfuron (als) and kanamycin resistance (NPT) genes Survey of 100, 000 seeds showed that perplant outcrossing rate was 0. 3% for the mutant fathers and 5. 98% for transgenic fathers

Direct non-target effects on beneficial/native organisms Species Toxin source Effects Monarch butterfly BT corn pollen (larva feeding on pollen) 44% death on Bt pollen (event 176) none on non-bt pollen. Same same (larva feeding on leaves dusted with pollen) 20% mortality on event. Bt 11 vs 3% control. Black swallow Tail butterfly Bt corn pollen (event 810, 176) No relation between pollen deposition (field test) and larval wt. or mortality. 2 -spot ladybeetle aphids colonizing transgenic GNA potato plants Lower fecundity, egg viability, adult longevity. Convergent Lady beetle same No effect Soil microbes GNA potato some transient effect on rhizosphere microbe Same glyphosate canola less diverse bacterial community of rhizosphere

Unwanted DNA in transgenic crops Transgenic plants often contain other DNA sequences in addition to the gene of interest: in the vector backbone lies E. coli antibiotic resistance gene and origin of replication, and in the construct lies plant selectable marker gene. Horizontal gene transfer: gene transfer across sexually incompatible species. Transfer of antibiotic resistance gene from plant to bacteria in the gut flora of animals will pose medical risk to human and cattle: if the antibiotic resistance gene escapes into bacterial cells, the drug would become useless for medical treatment of that animal. An example of horizontal gene transfer: Aquaporins (AQP) and/or aquaglyceroporin (GLP) occur in all organism. GLP are absent in plants, but one AQP located in tonoplast is capable of transporting glycerol just like GLP does. Molecular phylogenic study suggests that this plant glycerol transporter may have originated from a single event of horizontal gene transfer from bacteria to plant, ~1200 million years ago.