Membrane potential Resting membrane potential Action potential End

Membrane potential

• • Resting membrane potential Action potential End plate potential Synaptic potential Excitatory post synaptic potential Inhibitatory post synaptic potential
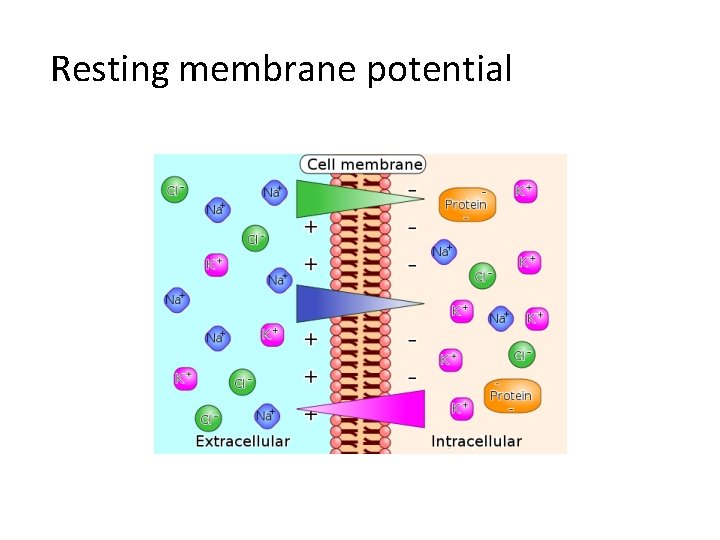
Resting membrane potential
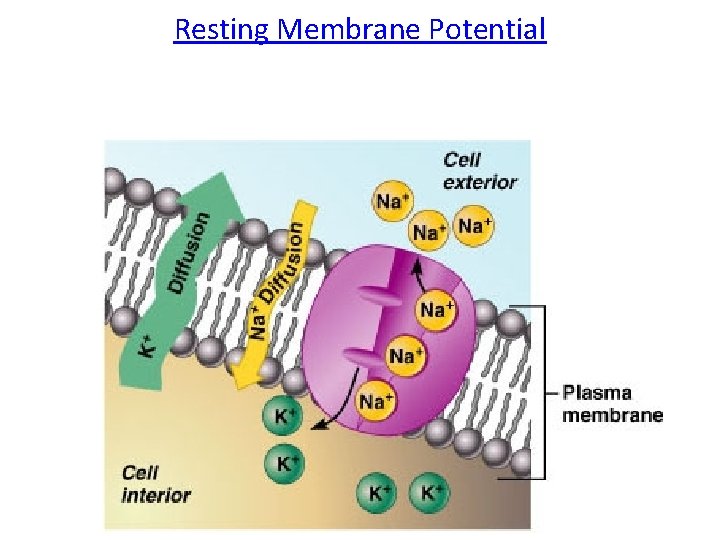
Resting Membrane Potential

GENESIS OF RESTING MEMBRANE POTENTIAL • PERMEABILITY OF Na +and K+ TO THE CELL MEMBRANE • Na +and K+ PUMP • NERNST POTENTIAL • Gibbs Donnan equilibrium potential • GOLDSMAN CONSTANT FIELD EQUATION
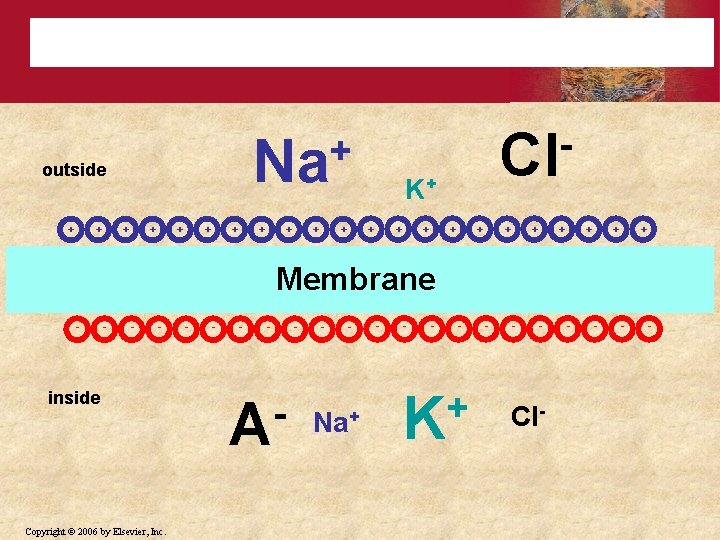
Resting Membrane Potential + + + K+ + + outside Cl + + Na + + + + Membrane - - Cl- - - + K - - Na+ - - - - - Copyright © 2006 by Elsevier, Inc. A - inside
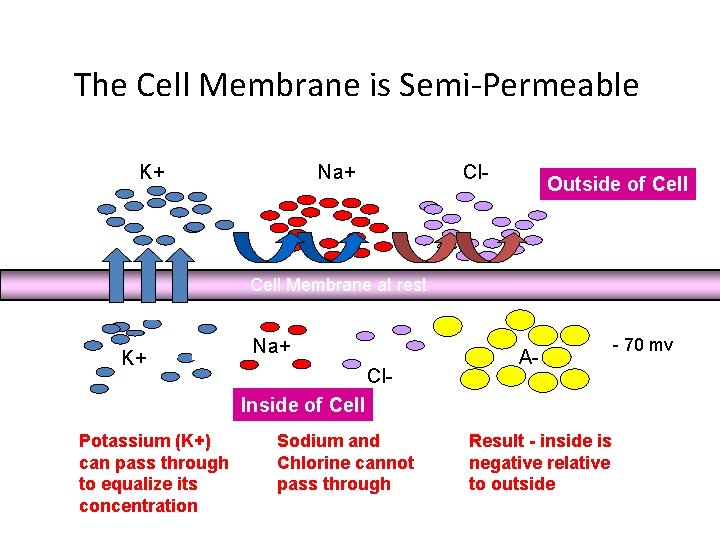
The Cell Membrane is Semi-Permeable K+ Na+ Cl- Outside of Cell Membrane at rest K+ Na+ Cl- A- Inside of Cell Potassium (K+) can pass through to equalize its concentration Sodium and Chlorine cannot pass through Result - inside is negative relative to outside - 70 mv

• Resting Membrane Potential (RMP) is the voltage (charge) difference across the cell membrane when the cell is at rest. • RMP is a product of the distribution of charged particles (ions). • The membrane potential is always negative inside the cell, and varies in size from – 20 to – 200 m. V (mill volt) in different cells and species (in humans it is – 70 m. V).
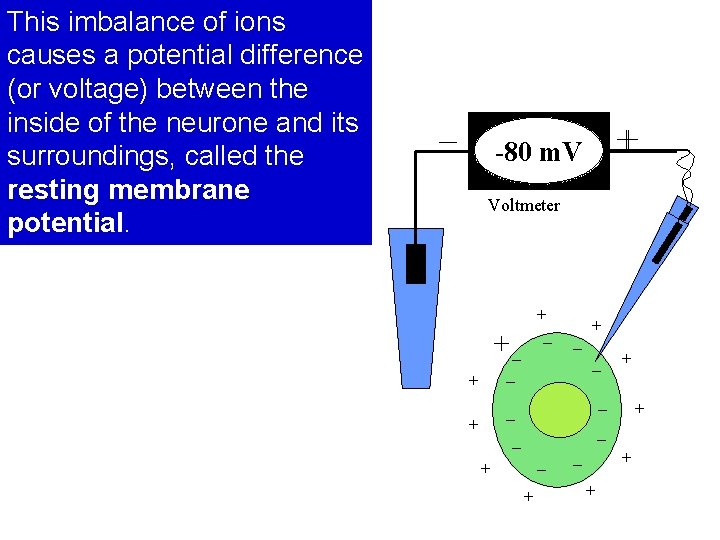
This imbalance of ions causes a potential difference (or voltage) between the inside of the neurone and its surroundings, called the resting membrane potential. – + -80 m. V Voltmeter + +– + – – – + – + + +
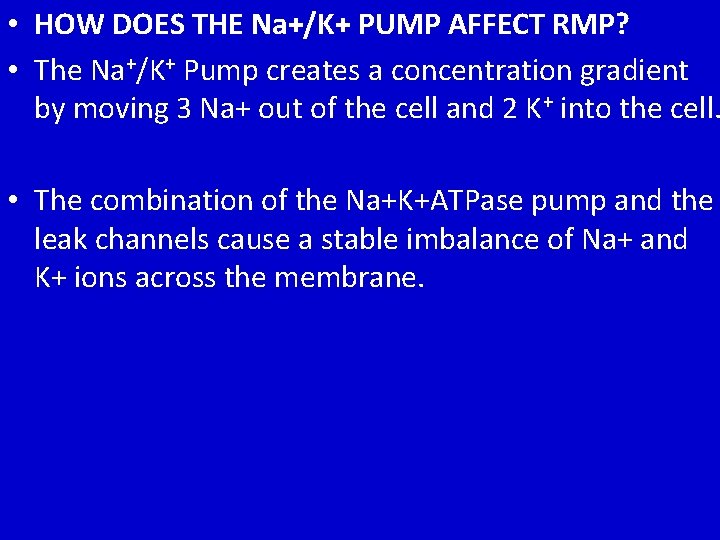
• HOW DOES THE Na+/K+ PUMP AFFECT RMP? • The Na+/K+ Pump creates a concentration gradient by moving 3 Na+ out of the cell and 2 K+ into the cell. • The combination of the Na+K+ATPase pump and the leak channels cause a stable imbalance of Na+ and K+ ions across the membrane.
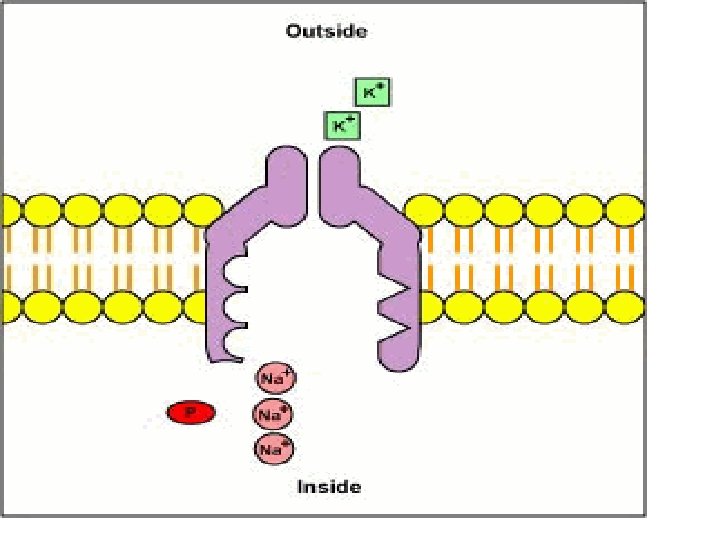

• Membrane Potential is always negative (-70 m. V) • K+ pass easily into the cell • Cl- and Na+ have a more difficult time crossing • Negatively charged protein molecules inside the neurone cannot pass the membrane
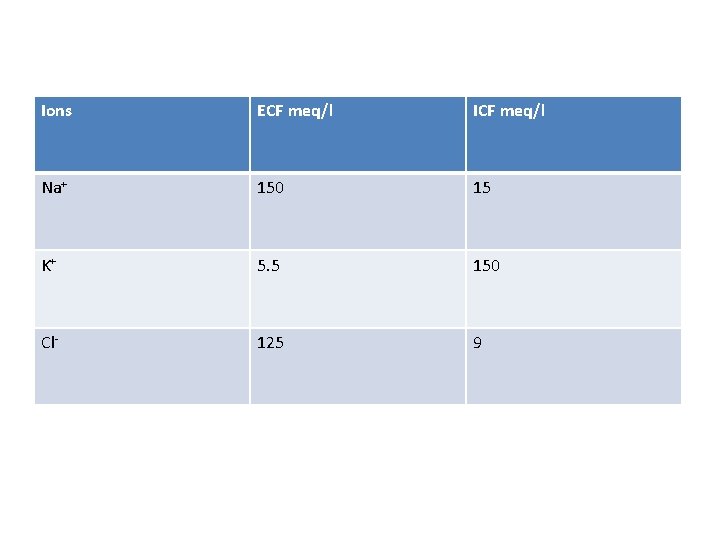
Ions ECF meq/l ICF meq/l Na+ 150 15 K+ 5. 5 150 Cl- 125 9
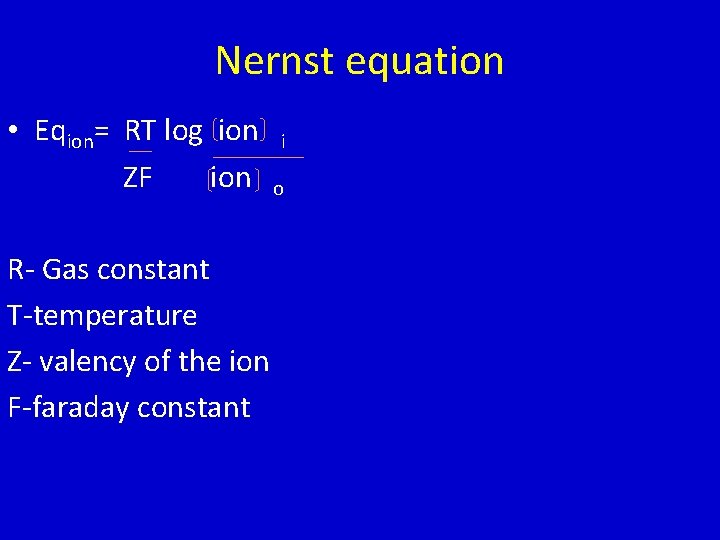
Nernst equation • Eqion= RT log ion i ZF ion o R- Gas constant T-temperature Z- valency of the ion F-faraday constant

• The equilibrium potential of a particular ion is usually designated by the notation Eion. • The equilibrium potential for any ion can be calculated using the Nernst equation. • EMF(millivolts) = ± 61 log Conc inside/Conc outside Equilibrium potential for Sodium (ENa) = +61 mv • Equilibrium potential for potassium(EK) =-94 mv • Equilibrium potential for chloride(Ecl)=-85 mv
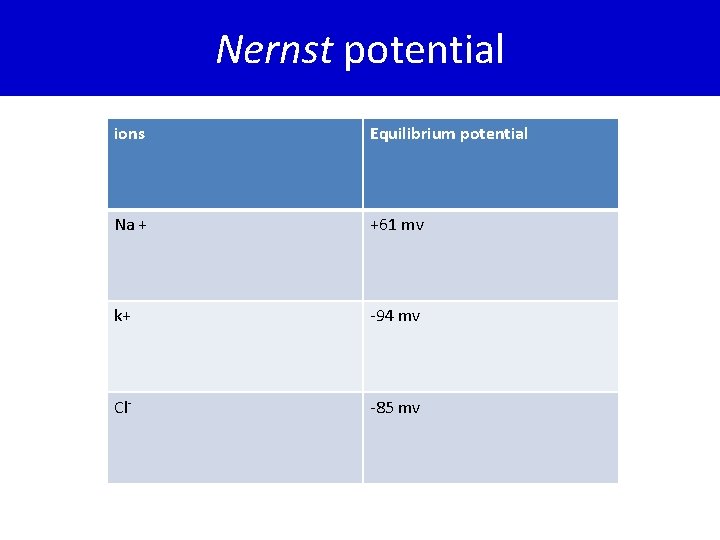
Nernst potential ions Equilibrium potential Na + +61 mv k+ -94 mv Cl- -85 mv
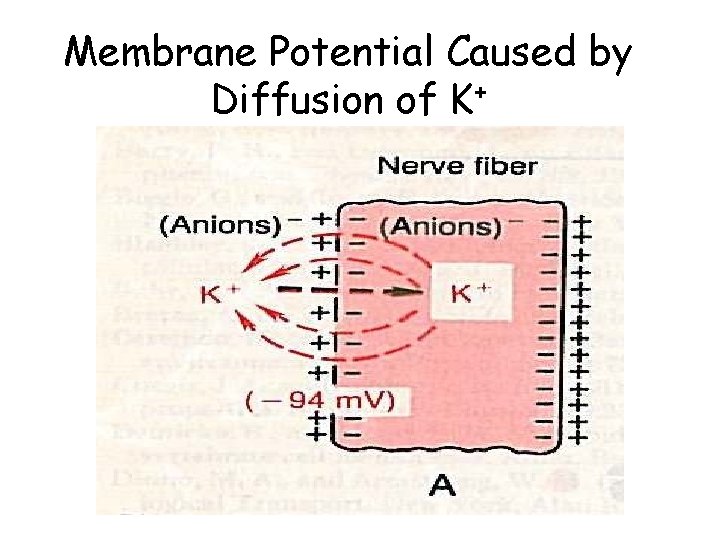
Membrane Potential Caused by Diffusion of K+
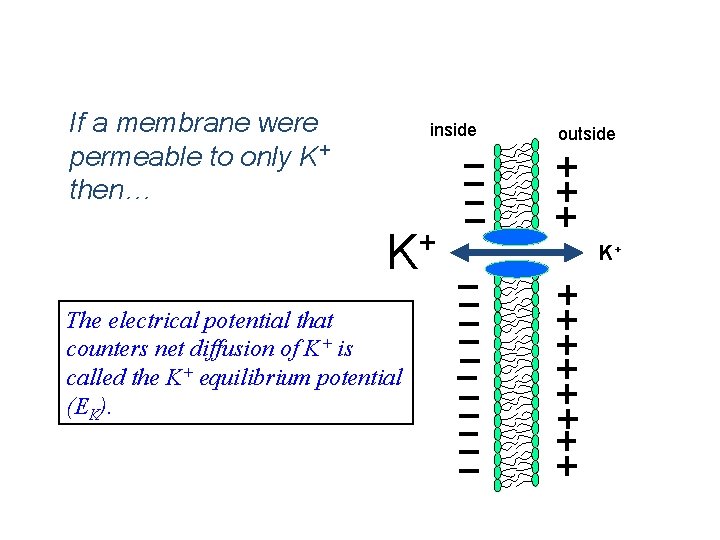
Simplest Case Scenario: If a membrane were permeable to only K+ then… inside + K The electrical potential that counters net diffusion of K+ is called the K+ equilibrium potential (EK). outside K+
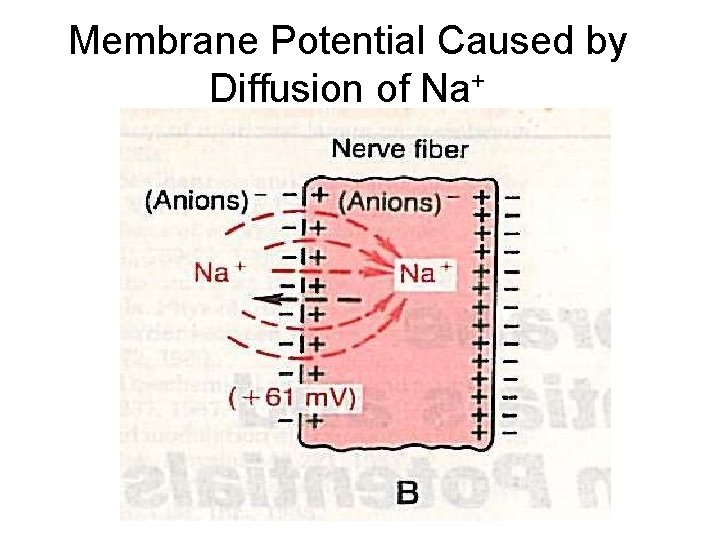
Membrane Potential Caused by Diffusion of Na+
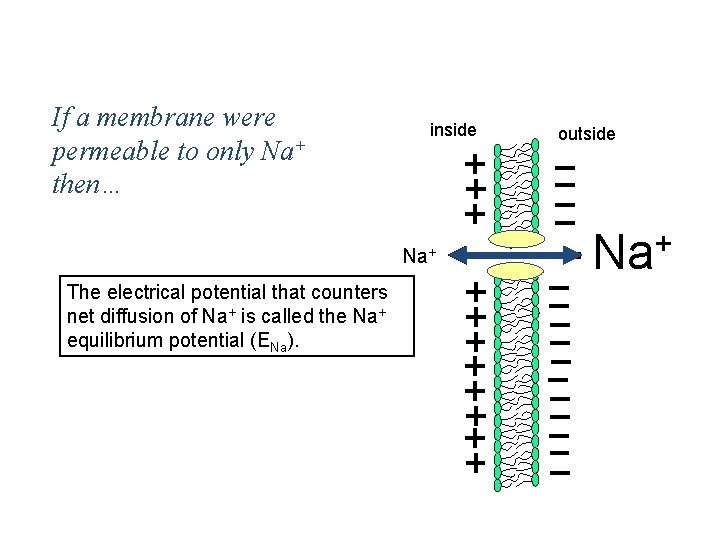
Simplest Case If a membrane were Scenario: permeable to only then… Na+ inside Na+ The electrical potential that counters net diffusion of Na+ is called the Na+ equilibrium potential (ENa). outside + Na
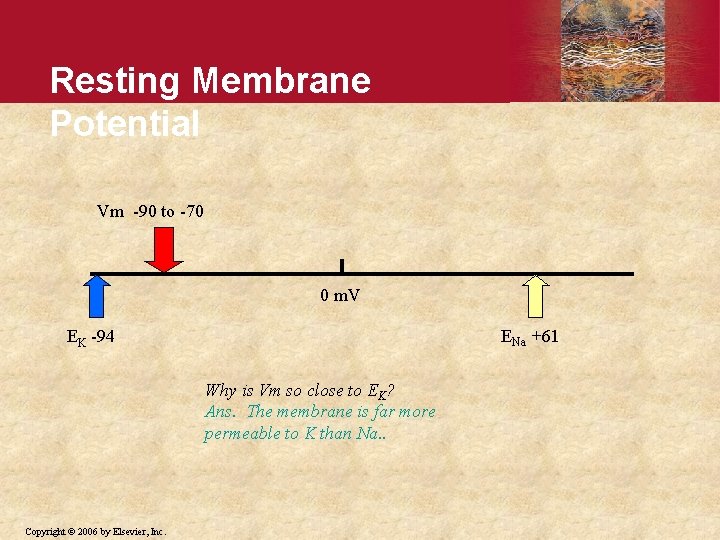
Resting Membrane Potential Vm -90 to -70 0 m. V ENa +61 EK -94 Why is Vm so close to EK? Ans. The membrane is far more permeable to K than Na. . Copyright © 2006 by Elsevier, Inc.
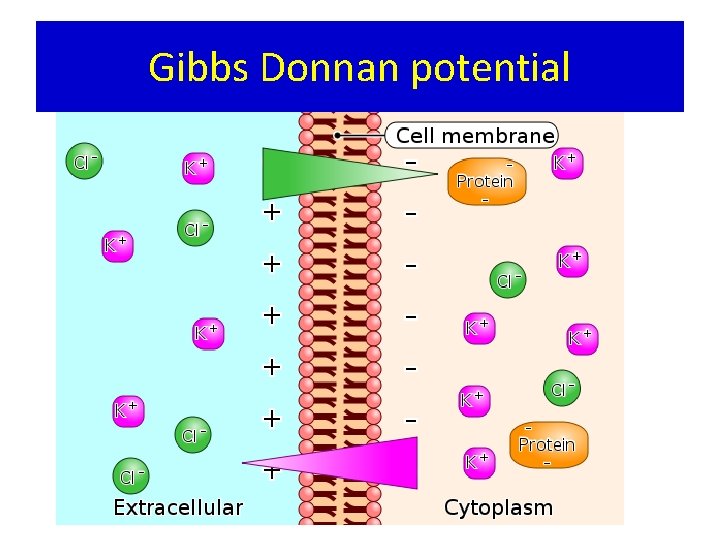
Gibbs Donnan potential
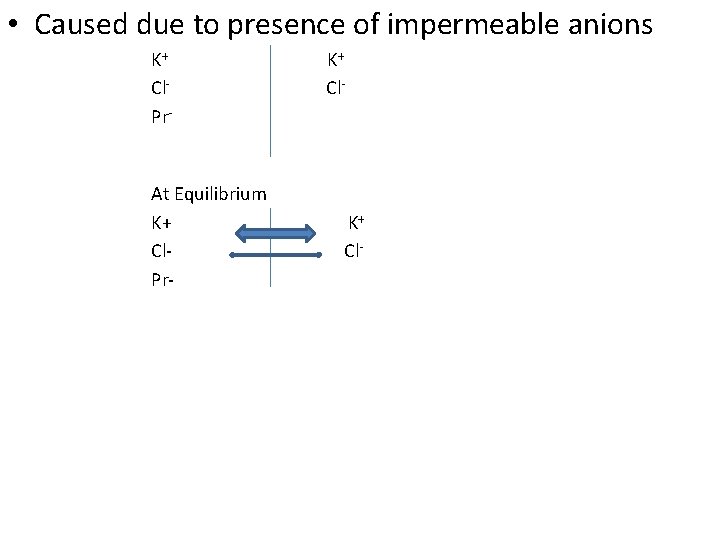
• Caused due to presence of impermeable anions K+ K+ Cl- Cl. Pr- At Equilibrium K+ Cl- Cl. Pr-
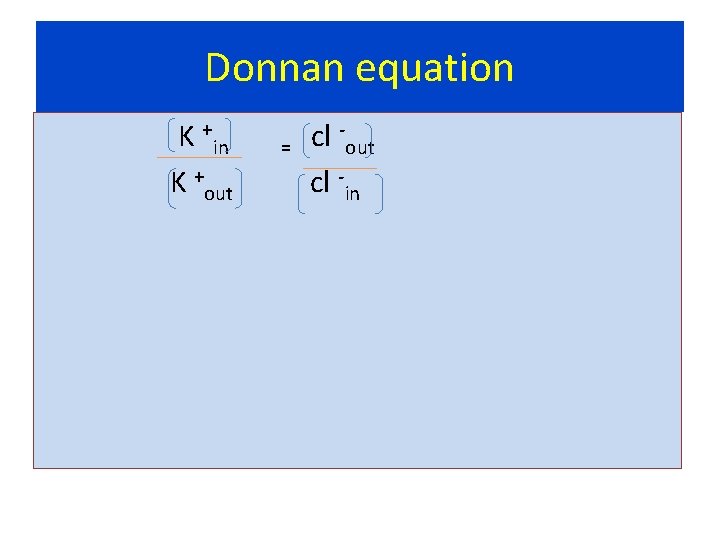
Donnan equation K +in = cl -out + K cl out in
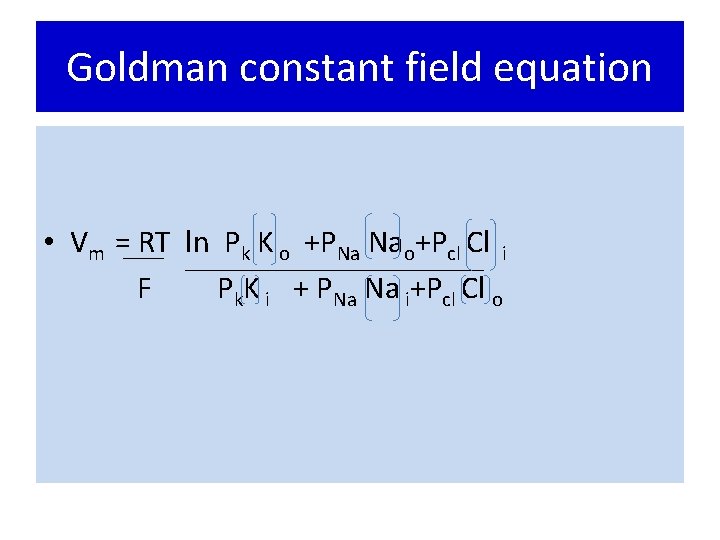
Goldman constant field equation • Vm = RT ln Pk K o +PNa Nao+Pcl Cl i F Pk. K i + PNa Na i+Pcl Cl o

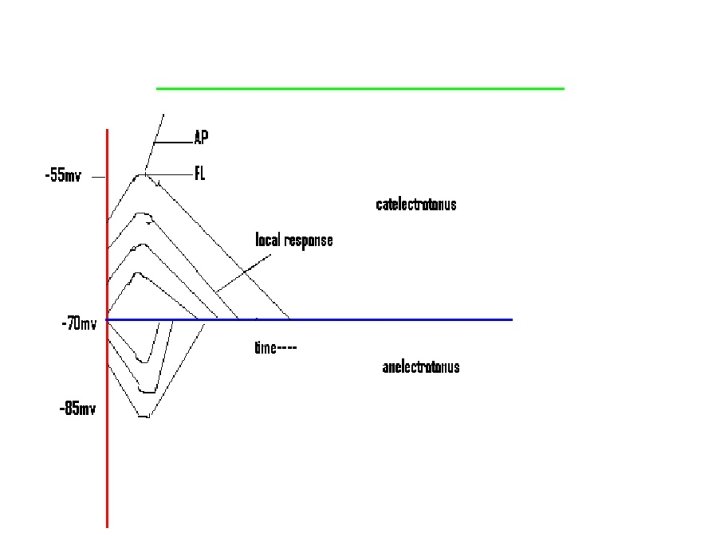
• Local response • Sub minimal stimulus is applied. • Axon is stimulated with slowly increasing strength of stimuli. MP is decreased by 7 mv. • Cathelectrotonic potential developed at the cathode. • Anelectrotonic potential is developed at the anode.
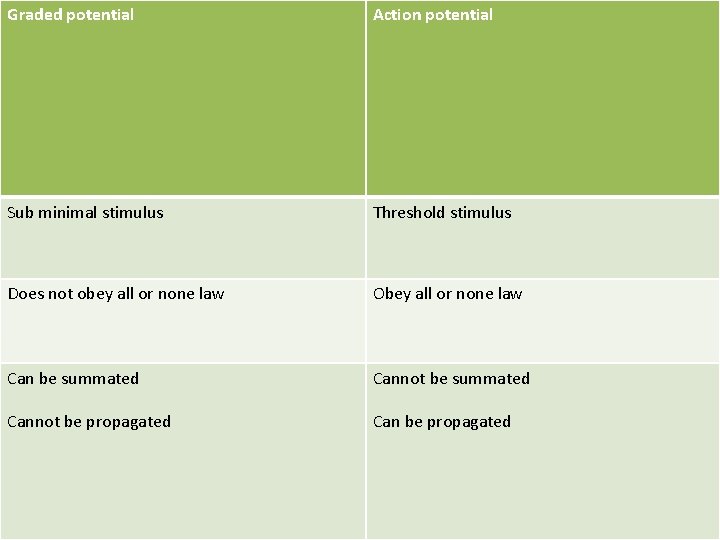
Graded potential Action potential Sub minimal stimulus Threshold stimulus Does not obey all or none law Obey all or none law Can be summated Cannot be summated Cannot be propagated Can be propagated
- Slides: 28