Potential diagrams v v v v Chemical potential






- Slides: 22

Potential diagrams v v v v Chemical potential, intensive and extensive parameters Types of phase diagrams Unary diagrams Binary diagrams Ternary diagrams Volatility diagram and Ellingham diagram Schreinemakers rules Fe Fe-O Al-Ni-O

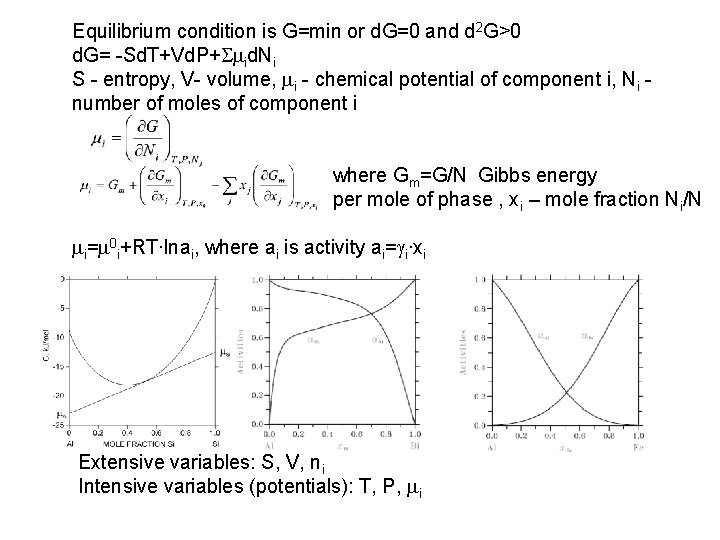
Equilibrium condition is G=min or d. G=0 and d 2 G>0 d. G= -Sd. T+Vd. P+Smid. Ni S - entropy, V- volume, mi - chemical potential of component i, Ni number of moles of component i where G m=G/N Gibbs energy per mole of phase , x i – mole fraction Ni/N mi=m 0 i+RT∙lnai, where ai is activity ai=gi∙xi Extensive variables: S, V, ni Intensive variables (potentials): T, P, mi

Types of diagrams: 1. Potential diagrams – two potentials 2. Mixed diagrams – one potential and one ratio of extensive properties 3. Extensive properties diagrams – two ratios of extensive properties Examples 1. P-T diagram, T-activity Fe Al-Mg 2. T-x diagram, vertical sections 3. Isothermal sections xi-xj, liquidus projections Fe-O Al-Mg-Si

Other classifications 1. Sections 2. Projections 1. Phase relations 2. Property diagrams How to control chemical potential experimentally? Equilibria in gas mixtures: 2 H 2+O 2=2 H 2 O DG 0+RTln(p. H 2 O)2/(p. O 2·p. H 22)=0 2 CO+O 2=2 CO 2 DG 0+RTln(p. CO 2)2/(p. O 2·p. CO 2)=0

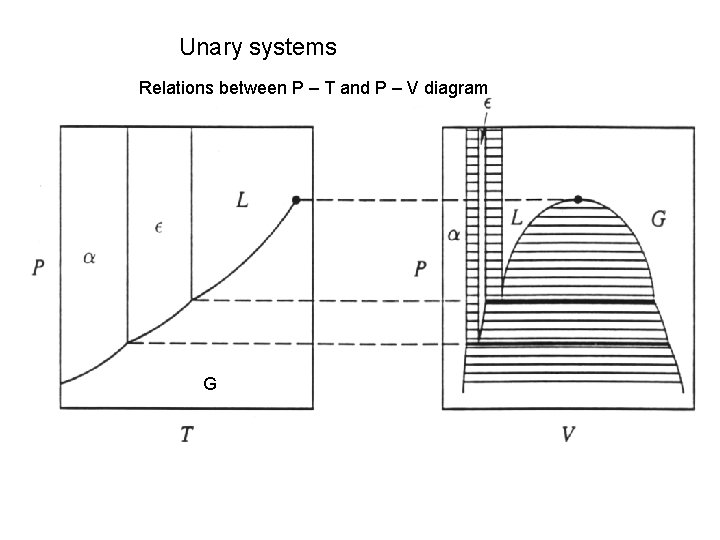
Unary systems Relations between P – T and P – V diagram G

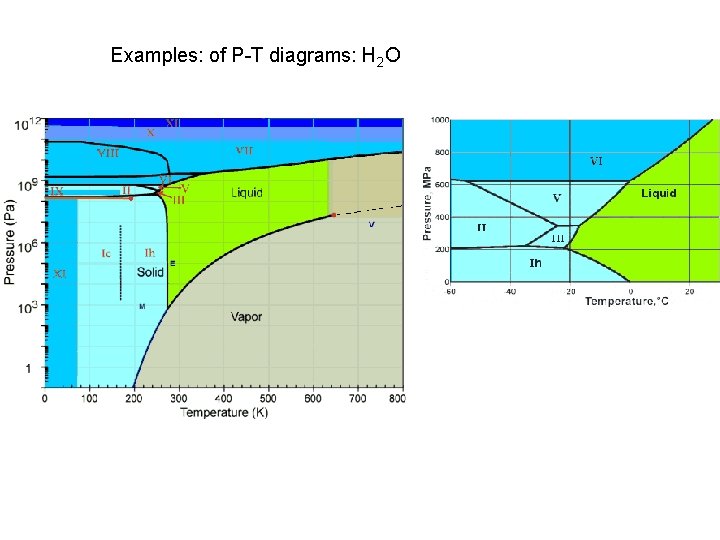
Examples: of P-T diagrams: H 2 O

Phase diagrams of Fe

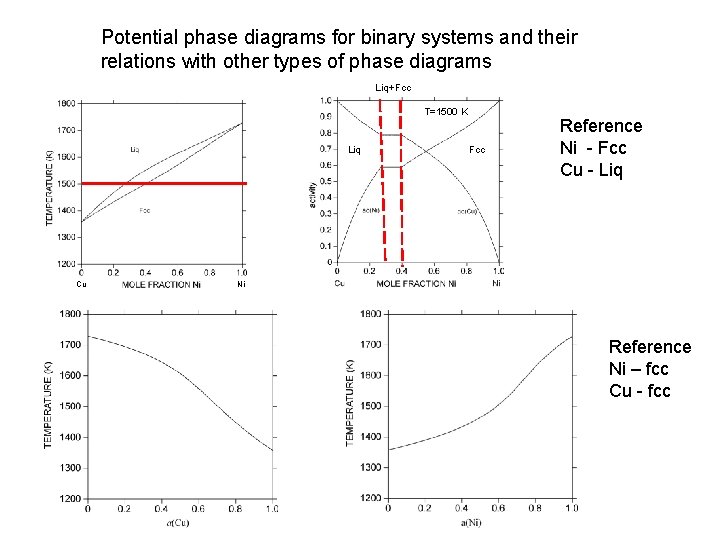
Potential phase diagrams for binary systems and their relations with other types of phase diagrams Liq+Fcc T=1500 K Liq Cu Fcc Reference Ni - Fcc Cu - Liq Ni Reference Ni – fcc Cu - fcc

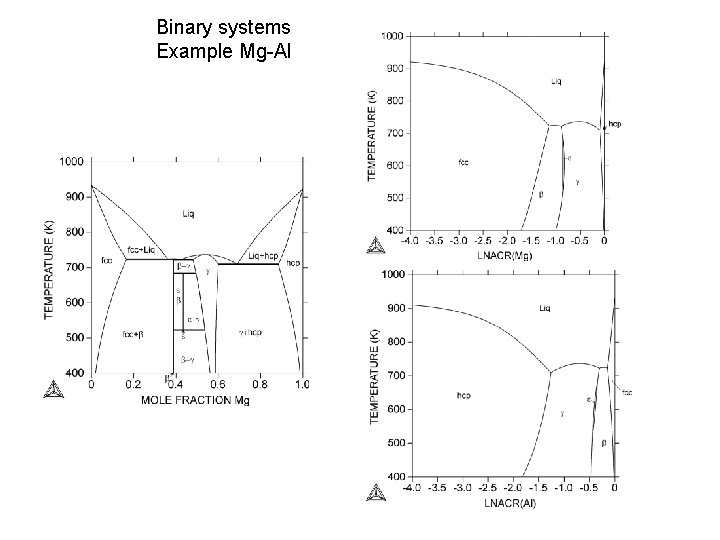
Binary systems Example Mg-Al

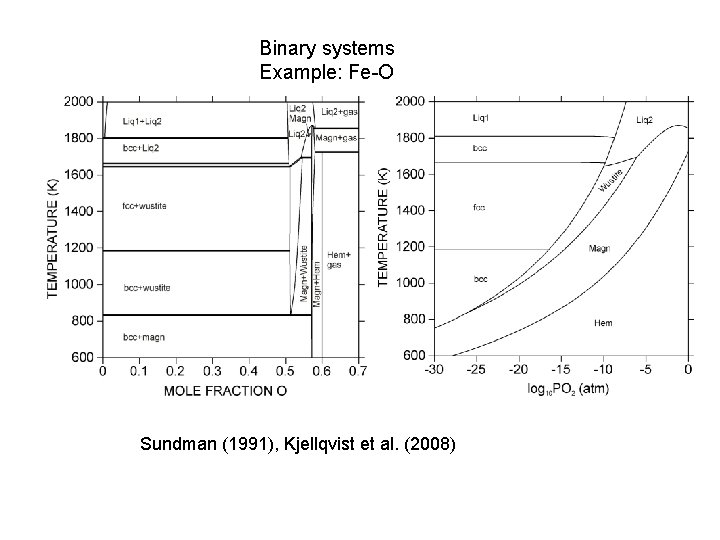
Binary systems Example: Fe-O Sundman (1991), Kjellqvist et al. (2008)

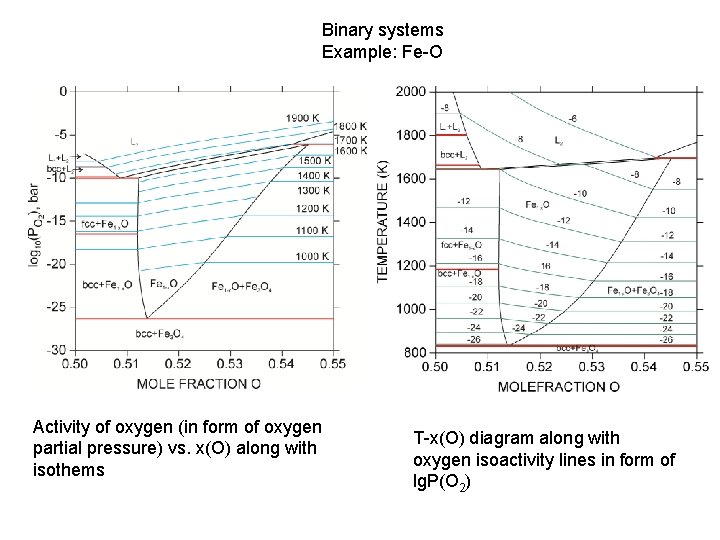
Binary systems Example: Fe-O Activity of oxygen (in form of oxygen partial pressure) vs. x(O) along with isothems T-x(O) diagram along with oxygen isoactivity lines in form of lg. P(O 2)

Ternary diagrams. Example Al-Ni-O: phase diagrams at 1100 K (c) a. Relations between activity of O and Ni b. Activity of O vs. Ratio Ni/(Ni+Al); 1 - Al 3 Ni 2, 2 – Al 3 Ni 2+b c. Isothermal section 1 - Al 2 O 3+Al 3 Ni 2, 2 – Al 2 O 3 -Al 3 Ni 2+b

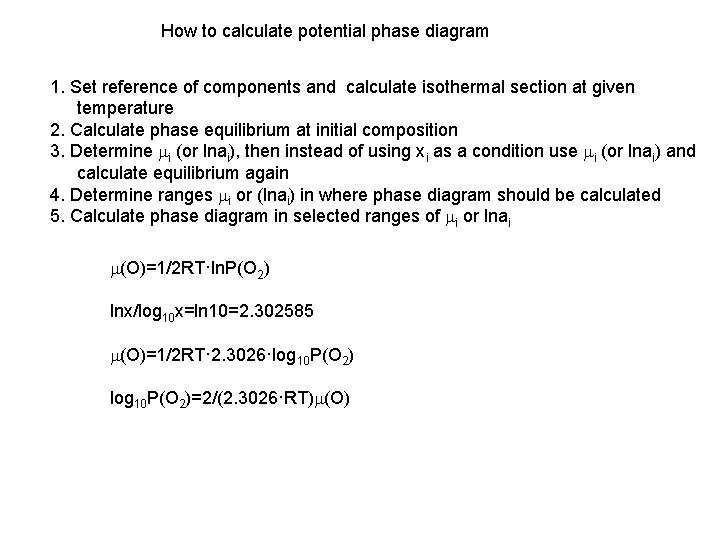
How to calculate potential phase diagram 1. Set reference of components and calculate isothermal section at given temperature 2. Calculate phase equilibrium at initial composition 3. Determine mi (or lnai), then instead of using xi as a condition use mi (or lnai) and calculate equilibrium again 4. Determine ranges mi or (lnai) in where phase diagram should be calculated 5. Calculate phase diagram in selected ranges of mi or lnai m(O)=1/2 RT·ln. P(O 2) lnx/log 10 x=ln 10=2. 302585 m(O)=1/2 RT· 2. 3026·log 10 P(O 2)=2/(2. 3026·RT)m(O)

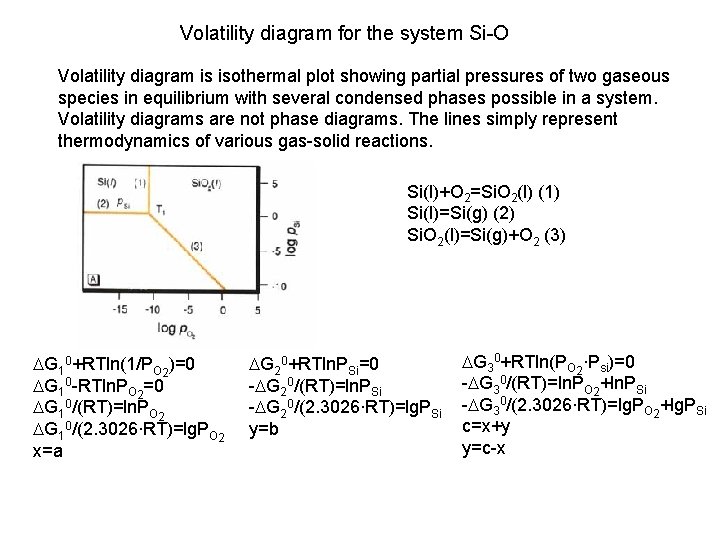
Volatility diagram for the system Si-O Volatility diagram is isothermal plot showing partial pressures of two gaseous species in equilibrium with several condensed phases possible in a system. Volatility diagrams are not phase diagrams. The lines simply represent thermodynamics of various gas-solid reactions. Si(l)+O 2=Si. O 2(l) (1) Si(l)=Si(g) (2) Si. O 2(l)=Si(g)+O 2 (3) DG 10+RTln(1/PO 2)=0 DG 10 -RTln. PO 2=0 DG 10/(RT)=ln. PO 2 DG 10/(2. 3026∙RT)=lg. PO 2 x=a DG 20+RTln. PSi=0 -DG 20/(RT)=ln. PSi -DG 20/(2. 3026∙RT)=lg. PSi y=b DG 30+RTln(PO 2∙Psi)=0 -DG 30/(RT)=ln. PO 2+ln. PSi -DG 30/(2. 3026∙RT)=lg. PO 2+lg. PSi c=x+y y=c-x

Volatility diagram for the Si-O system at 1900 K Minor species are neglected; maximum equilibrium pressure lines lg. K Si(l)+O 2=Si. O 2(l) (1) 15. 68 Si(l)=Si(g) (2) -4. 92 Si. O 2(l)=Si(g)+O 2 (3) -20. 6 2 Si(l)+O 2=2 Si. O(g) (4) 13. 86 2 Si. O 2(l)=2 Si. O(g)+O 2 (5) Si. O 2(l)+Si(l)=2 Si. O(g) (6) Si(l)+O 2=Si. O 2(g) (7) Si. O 2(l)=Si. O 2(g) (8) lg. K -17. 5 -1. 82 8. 29 -7. 3

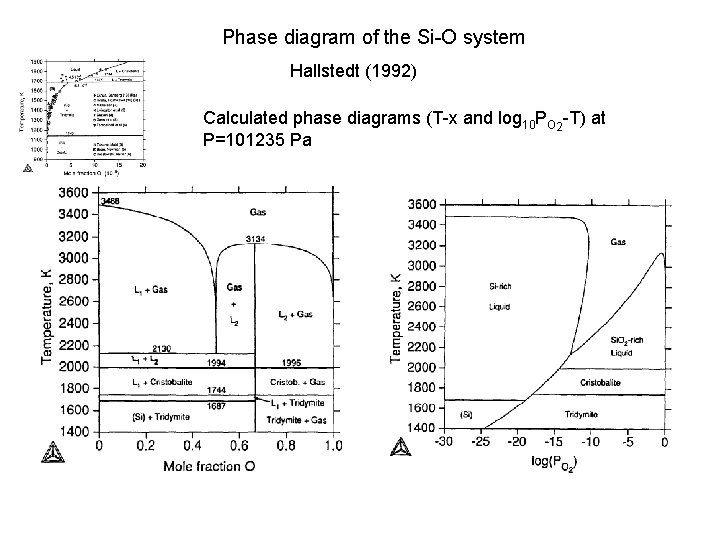
Phase diagram of the Si-O system Hallstedt (1992) Calculated phase diagrams (T-x and log 10 PO 2 -T) at P=101235 Pa

Ellingham type diagram A major application of Ellingham diagram is the determination of the conditions required to reduce metal compounds (i. e. oxides) to obtain pure metal.

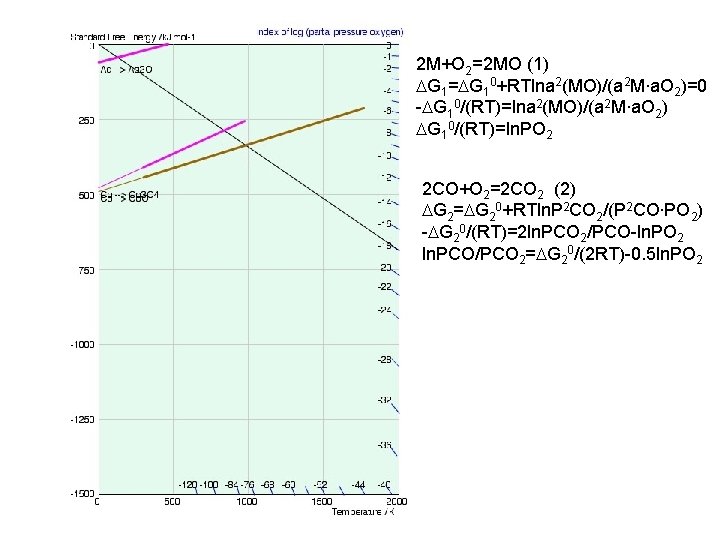
2 M+O 2=2 MO (1) DG 1=DG 10+RTlna 2(MO)/(a 2 M∙a. O 2)=0 -DG 10/(RT)=lna 2(MO)/(a 2 M∙a. O 2) DG 10/(RT)=ln. PO 2 2 CO+O 2=2 CO 2 (2) DG 2=DG 20+RTln. P 2 CO 2/(P 2 CO∙PO 2) -DG 20/(RT)=2 ln. PCO 2/PCO-ln. PO 2 ln. PCO/PCO 2=DG 20/(2 RT)-0. 5 ln. PO 2

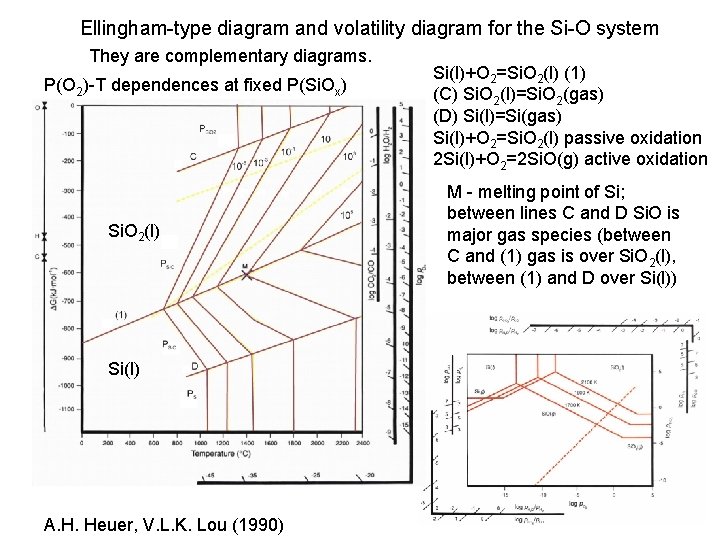
Ellingham-type diagram and volatility diagram for the Si-O system They are complementary diagrams. P(O 2)-T dependences at fixed P(Si. Ox) Si. O 2(l) Si(l) A. H. Heuer, V. L. K. Lou (1990) Si(l)+O 2=Si. O 2(l) (1) (C) Si. O 2(l)=Si. O 2(gas) (D) Si(l)=Si(gas) Si(l)+O 2=Si. O 2(l) passive oxidation 2 Si(l)+O 2=2 Si. O(g) active oxidation M - melting point of Si; between lines C and D Si. O is major gas species (between C and (1) gas is over Si. O 2(l), between (1) and D over Si(l))

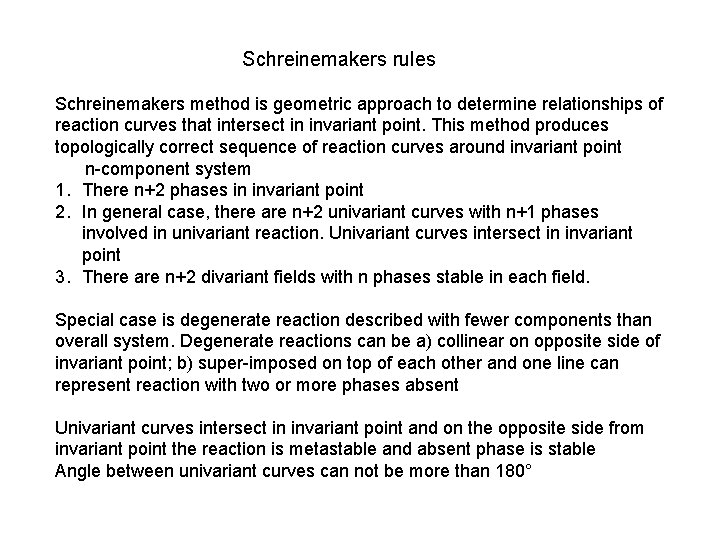
Schreinemakers rules Schreinemakers method is geometric approach to determine relationships of reaction curves that intersect in invariant point. This method produces topologically correct sequence of reaction curves around invariant point n-component system 1. There n+2 phases in invariant point 2. In general case, there are n+2 univariant curves with n+1 phases involved in univariant reaction. Univariant curves intersect in invariant point 3. There are n+2 divariant fields with n phases stable in each field. Special case is degenerate reaction described with fewer components than overall system. Degenerate reactions can be a) collinear on opposite side of invariant point; b) super-imposed on top of each other and one line can represent reaction with two or more phases absent Univariant curves intersect in invariant point and on the opposite side from invariant point the reaction is metastable and absent phase is stable Angle between univariant curves can not be more than 180°

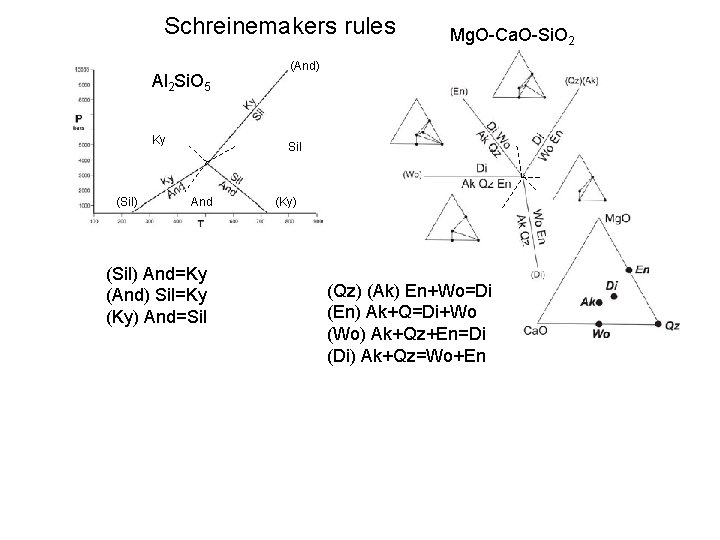
Schreinemakers rules Al 2 Si. O 5 Ky (Sil) Mg. O-Ca. O-Si. O 2 (And) Sil And (Sil) And=Ky (And) Sil=Ky (Ky) And=Sil (Ky) (Qz) (Ak) En+Wo=Di (En) Ak+Q=Di+Wo (Wo) Ak+Qz+En=Di (Di) Ak+Qz=Wo+En

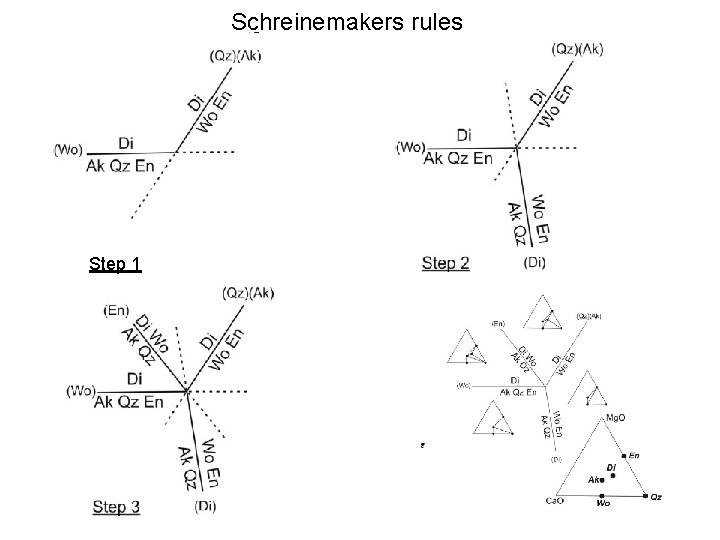
Schreinemakers rules Step 1