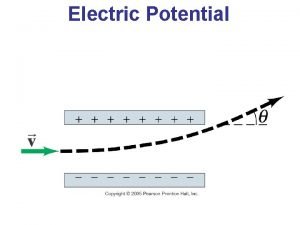
Potential Energy Diagrams Potential Energy Diagram Setup The



- Slides: 24

Potential Energy Diagrams

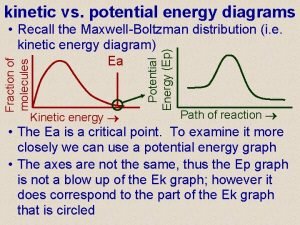
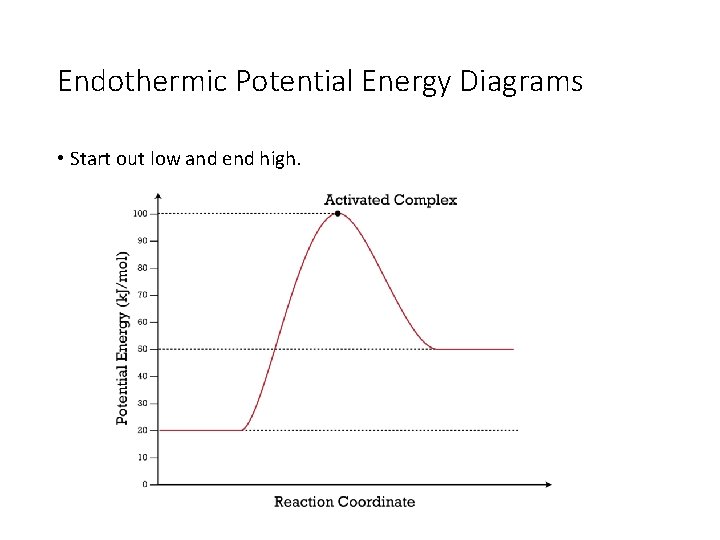
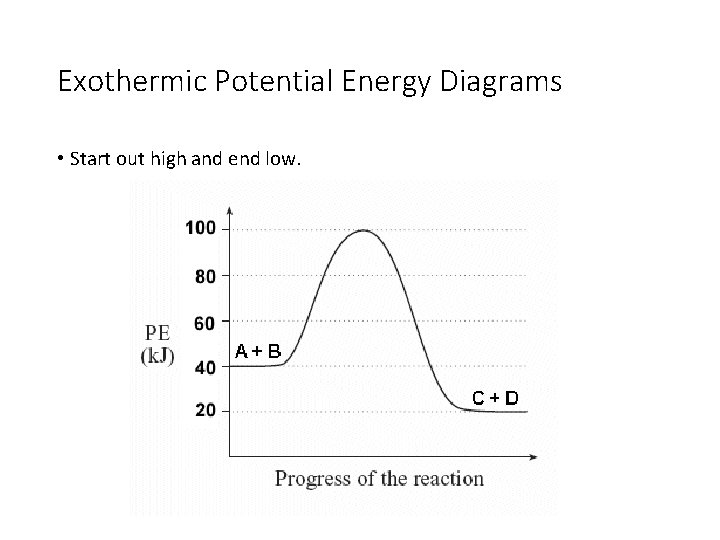
Potential Energy Diagram Setup • The x-axis gets labeled “reaction coordinate” or “reaction progress. ” • The y-axis gets labeled “potential energy. ” It usually has units of k. J (kilojoules). • These diagrams show you how the potential energy is changing and what’s happening inside the reaction as time goes on.

Endothermic Potential Energy Diagrams • Start out low and end high.

Exothermic Potential Energy Diagrams • Start out high and end low.

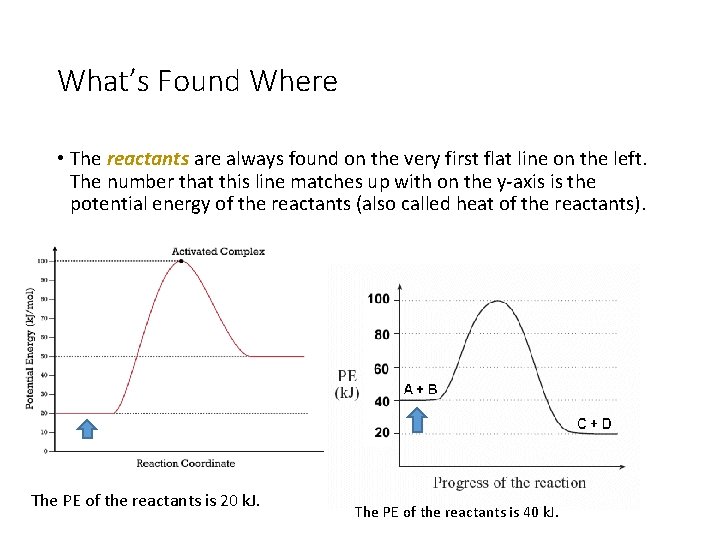
What’s Found Where • The reactants are always found on the very first flat line on the left. The number that this line matches up with on the y-axis is the potential energy of the reactants (also called heat of the reactants). The PE of the reactants is 20 k. J. The PE of the reactants is 40 k. J.

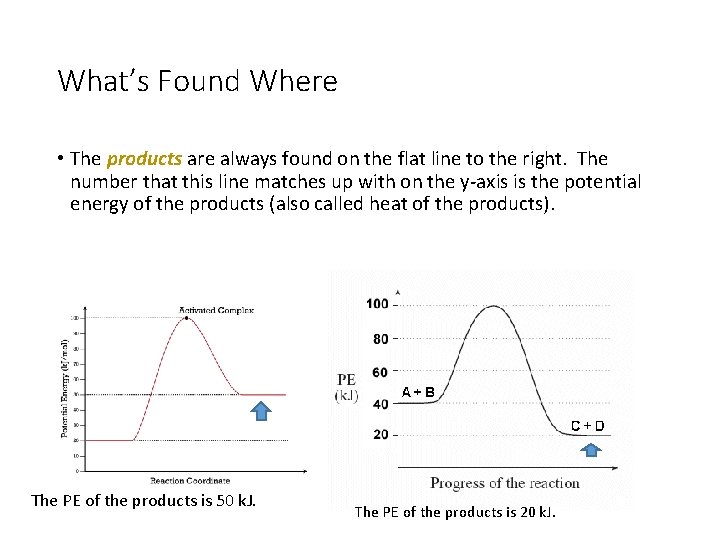
What’s Found Where • The products are always found on the flat line to the right. The number that this line matches up with on the y-axis is the potential energy of the products (also called heat of the products). The PE of the products is 50 k. J. The PE of the products is 20 k. J.

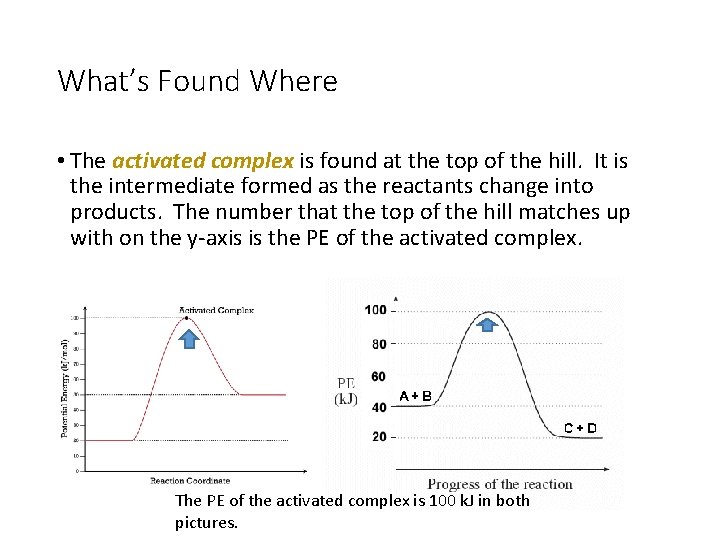
What’s Found Where • The activated complex is found at the top of the hill. It is the intermediate formed as the reactants change into products. The number that the top of the hill matches up with on the y-axis is the PE of the activated complex. The PE of the activated complex is 100 k. J in both pictures.

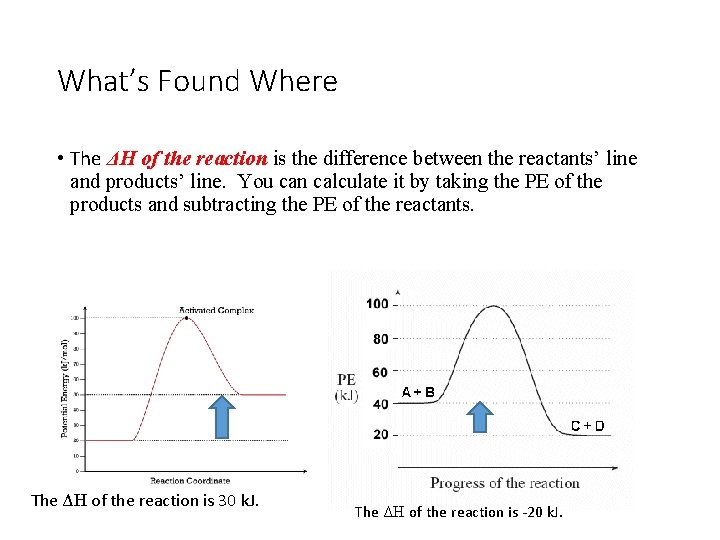
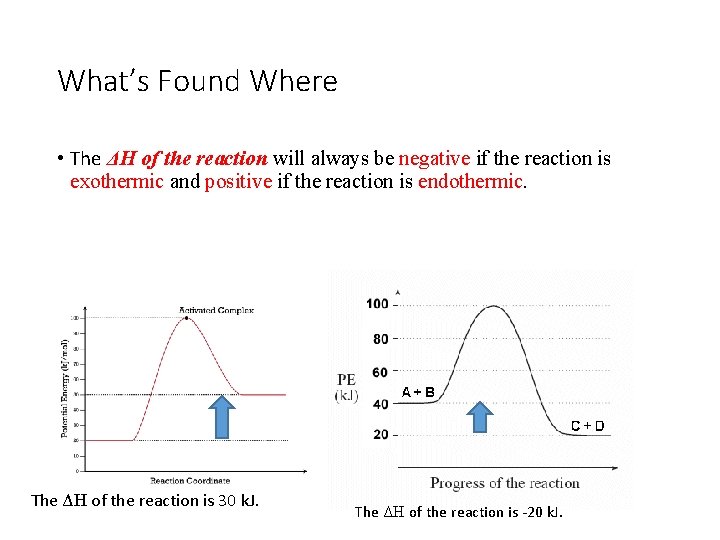
What’s Found Where • The ΔH of the reaction is the difference between the reactants’ line and products’ line. You can calculate it by taking the PE of the products and subtracting the PE of the reactants. The ΔH of the reaction is 30 k. J. The ΔH of the reaction is -20 k. J.

What’s Found Where • The ΔH of the reaction will always be negative if the reaction is exothermic and positive if the reaction is endothermic. The ΔH of the reaction is 30 k. J. The ΔH of the reaction is -20 k. J.

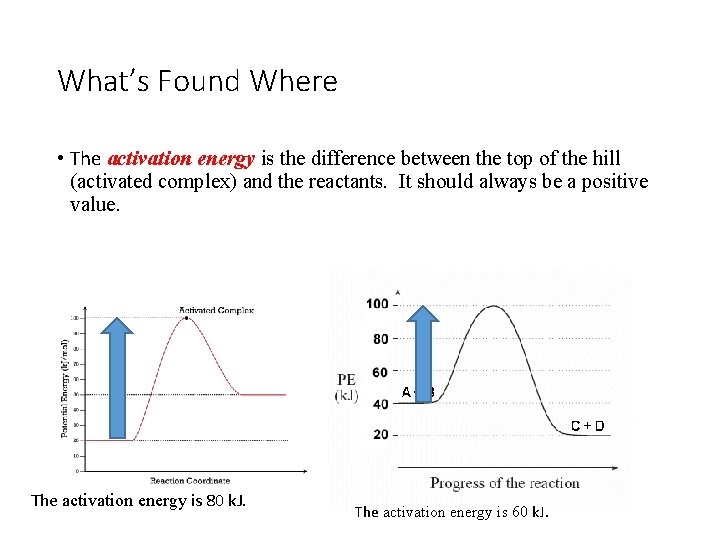
What’s Found Where • The activation energy is the difference between the top of the hill (activated complex) and the reactants. It should always be a positive value. The activation energy is 80 k. J. The activation energy is 60 k. J.

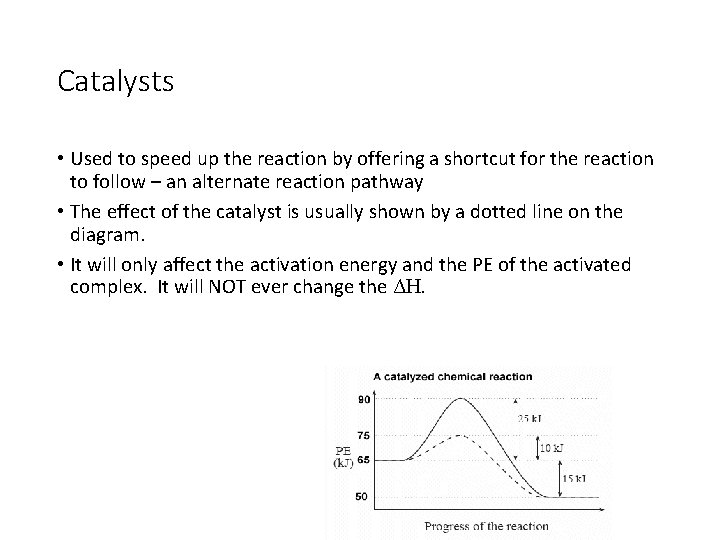
Catalysts • Used to speed up the reaction by offering a shortcut for the reaction to follow – an alternate reaction pathway • The effect of the catalyst is usually shown by a dotted line on the diagram. • It will only affect the activation energy and the PE of the activated complex. It will NOT ever change the ΔH.

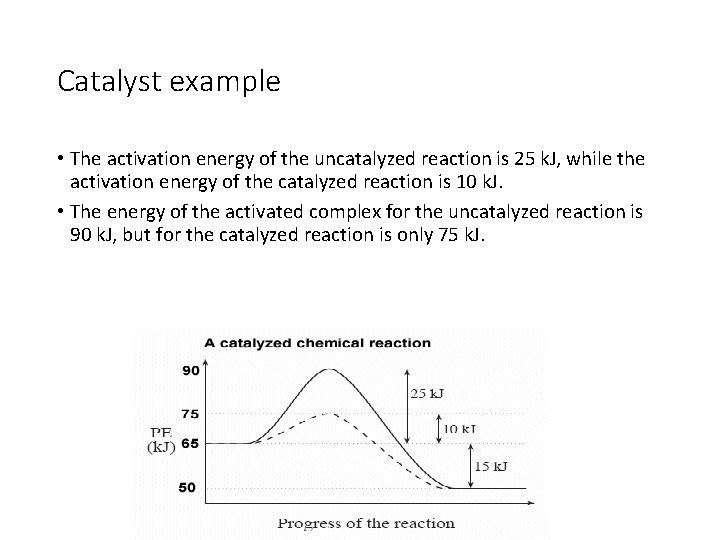
Catalyst example • The activation energy of the uncatalyzed reaction is 25 k. J, while the activation energy of the catalyzed reaction is 10 k. J. • The energy of the activated complex for the uncatalyzed reaction is 90 k. J, but for the catalyzed reaction is only 75 k. J.

Forward Vs. Reverse • If you are told the reaction is proceeding in the forward direction, you are following the diagram from left to right – just like we’ve been doing. • Sometimes, you are told that the reaction is proceeding in the reverse direction. In that case, you follow the reaction from right to left. What used to be your products are now your reactants.

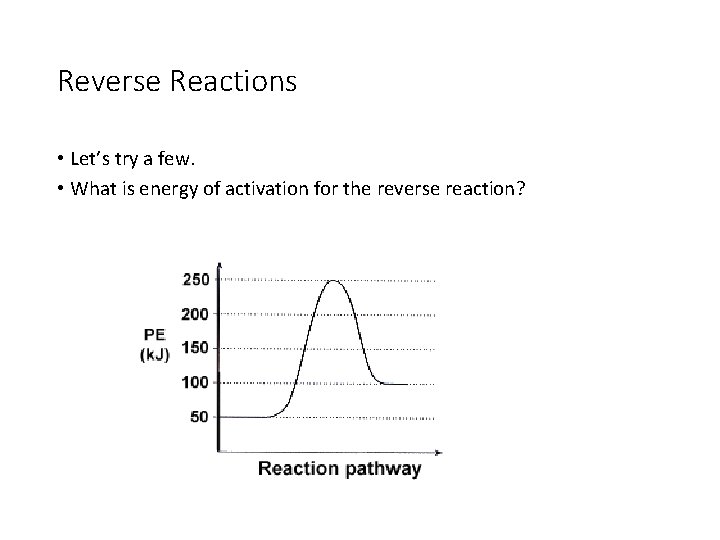
Reverse Reactions • Let’s try a few. • What is energy of activation for the reverse reaction?

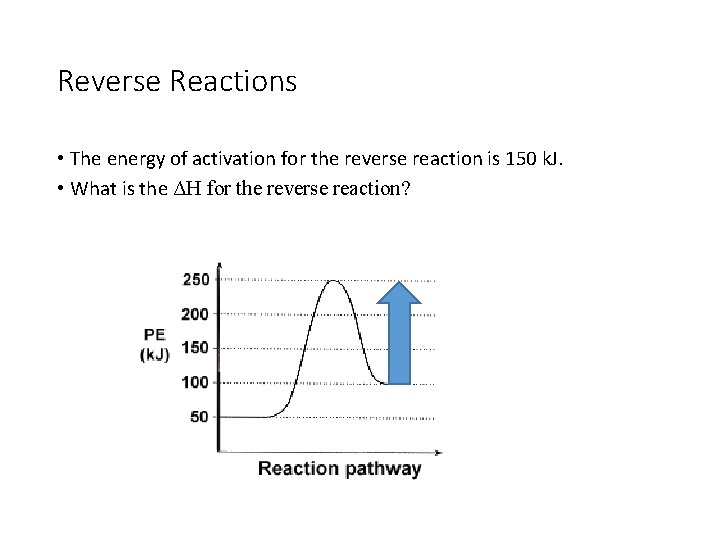
Reverse Reactions • The energy of activation for the reverse reaction is 150 k. J. • What is the ΔH for the reverse reaction?

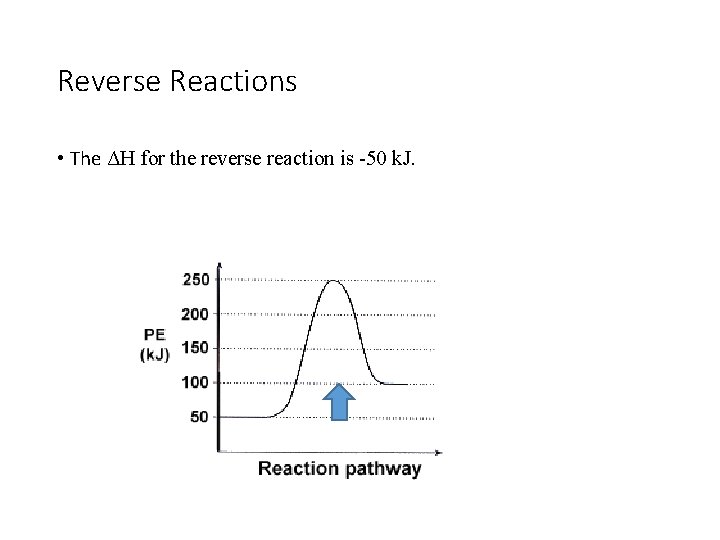
Reverse Reactions • The ΔH for the reverse reaction is -50 k. J.

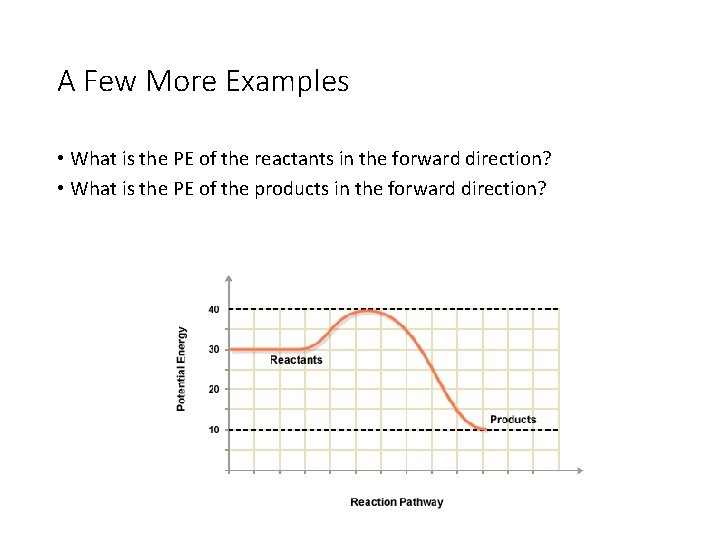
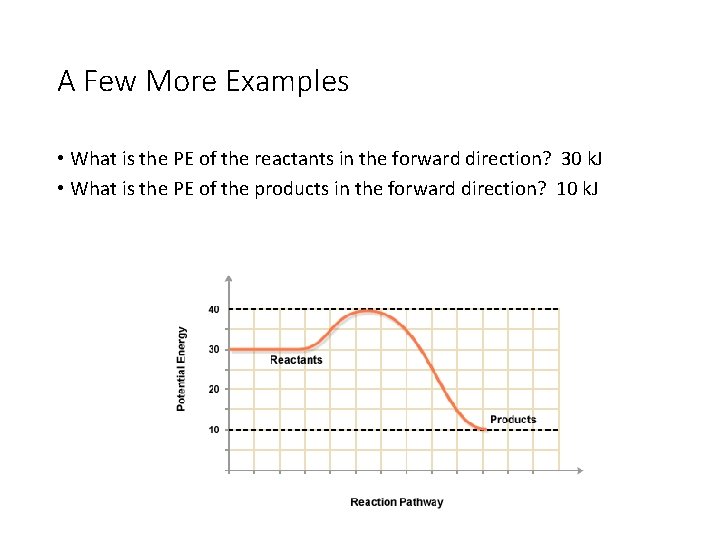
A Few More Examples • What is the PE of the reactants in the forward direction? • What is the PE of the products in the forward direction?

A Few More Examples • What is the PE of the reactants in the forward direction? 30 k. J • What is the PE of the products in the forward direction? 10 k. J

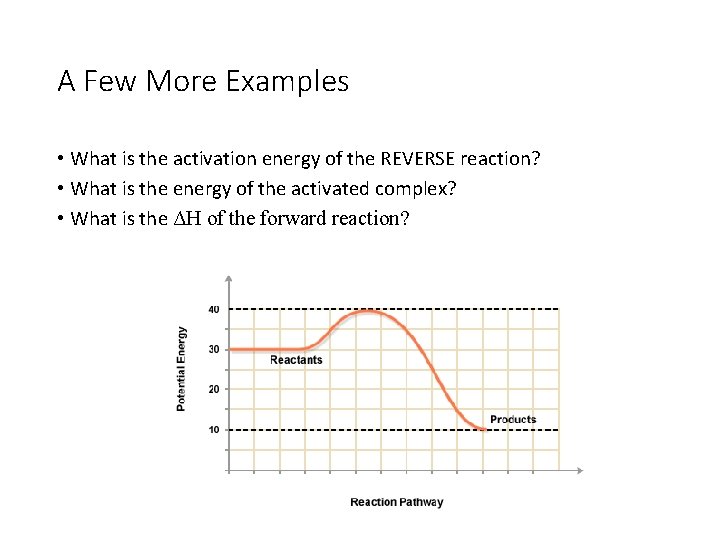
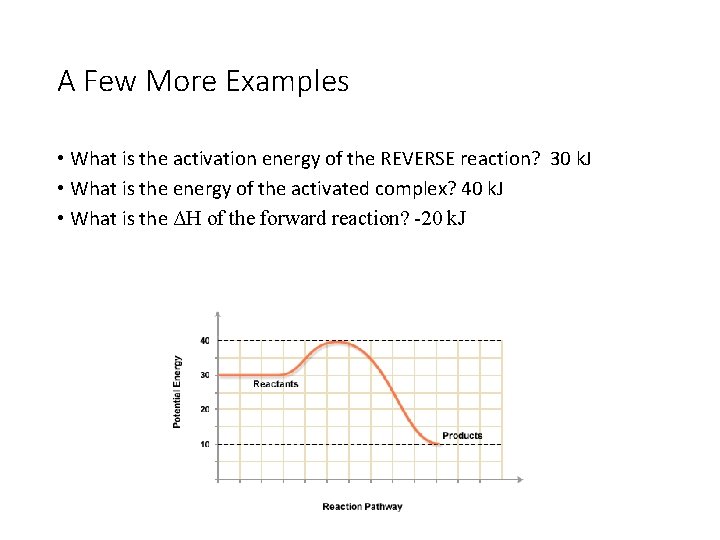
A Few More Examples • What is the activation energy of the REVERSE reaction? • What is the energy of the activated complex? • What is the ΔH of the forward reaction?

A Few More Examples • What is the activation energy of the REVERSE reaction? 30 k. J • What is the energy of the activated complex? 40 k. J • What is the ΔH of the forward reaction? -20 k. J

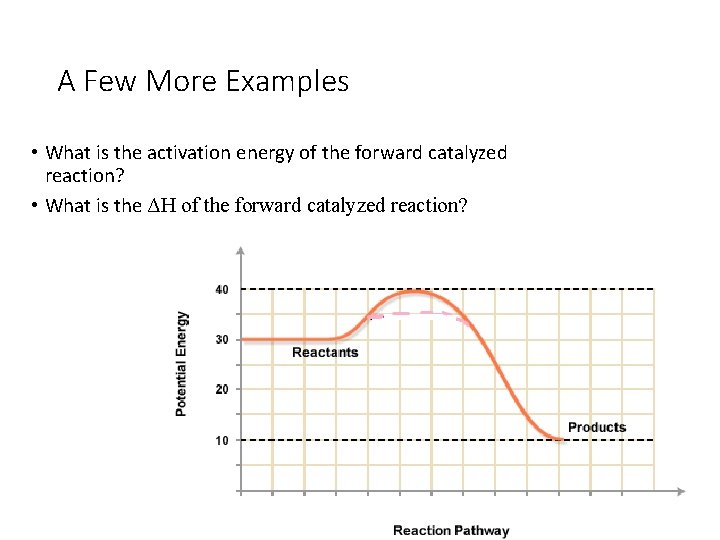
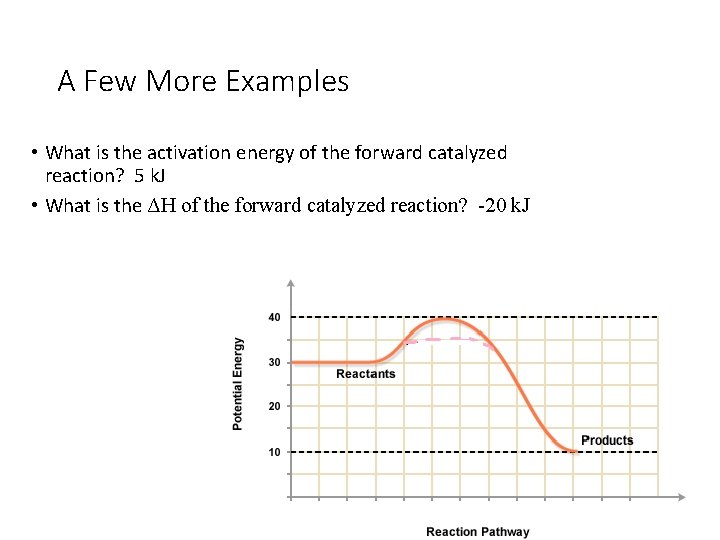
A Few More Examples • What is the activation energy of the forward catalyzed reaction? • What is the ΔH of the forward catalyzed reaction?

A Few More Examples • What is the activation energy of the forward catalyzed reaction? 5 k. J • What is the ΔH of the forward catalyzed reaction? -20 k. J

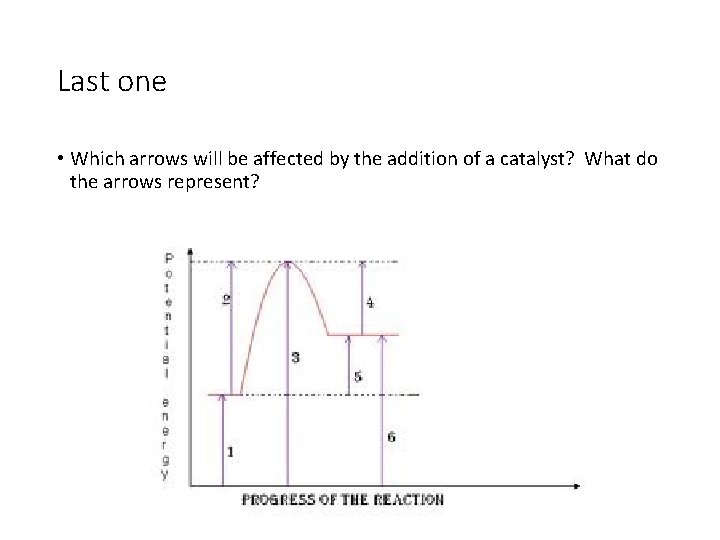
Last one • Which arrows will be affected by the addition of a catalyst? What do the arrows represent?

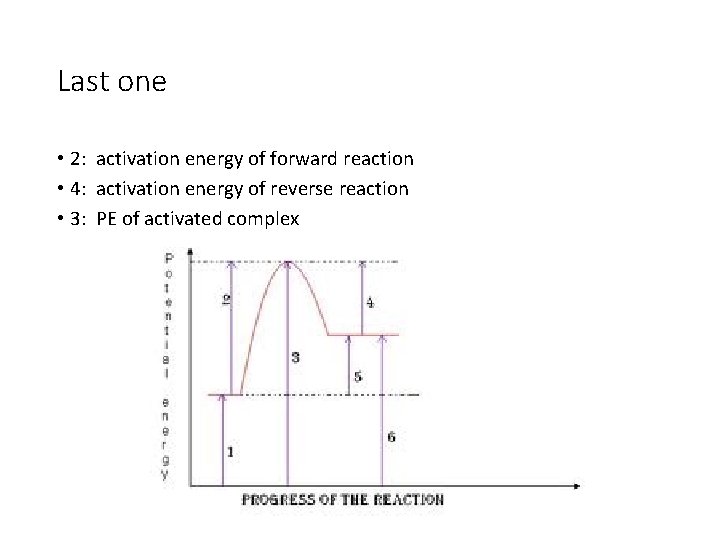
Last one • 2: activation energy of forward reaction • 4: activation energy of reverse reaction • 3: PE of activated complex
 Pe diagram
Pe diagram Use case model
Use case model Rake symbol in activity diagram
Rake symbol in activity diagram Unit of potential
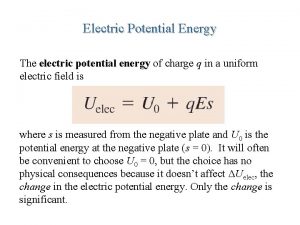
Unit of potential Potential energy of an electric field
Potential energy of an electric field Peelectric
Peelectric Formula energy
Formula energy Formula for total mechanical energy
Formula for total mechanical energy Potential energy examples
Potential energy examples Example of mechanical energy
Example of mechanical energy Gravitational potential energy vs kinetic energy
Gravitational potential energy vs kinetic energy Kinetic energy vs potential energy
Kinetic energy vs potential energy Kinetic and potential energy
Kinetic and potential energy Kinetic energy of spring
Kinetic energy of spring Formula of potential energy
Formula of potential energy Kinetic energy and potential energy formula
Kinetic energy and potential energy formula Enthalpy change in a potential energy diagram
Enthalpy change in a potential energy diagram Energy diagram
Energy diagram Potential and kinetic energy diagram
Potential and kinetic energy diagram Potential energy diagram heat of reaction
Potential energy diagram heat of reaction Activation complex
Activation complex Diagram of energy transformation
Diagram of energy transformation Eth eph ech
Eth eph ech Light bulb energy transfer diagram
Light bulb energy transfer diagram Forms of energy
Forms of energy