Polar or NonPolar That is the question Review






- Slides: 21

Polar or Non-Polar That is the question…

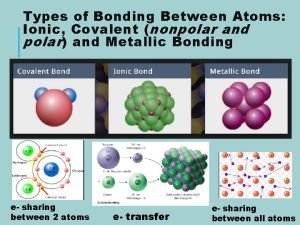
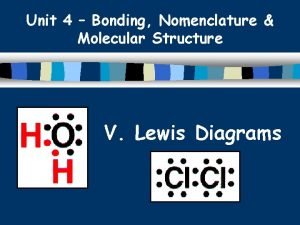
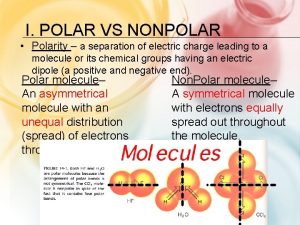
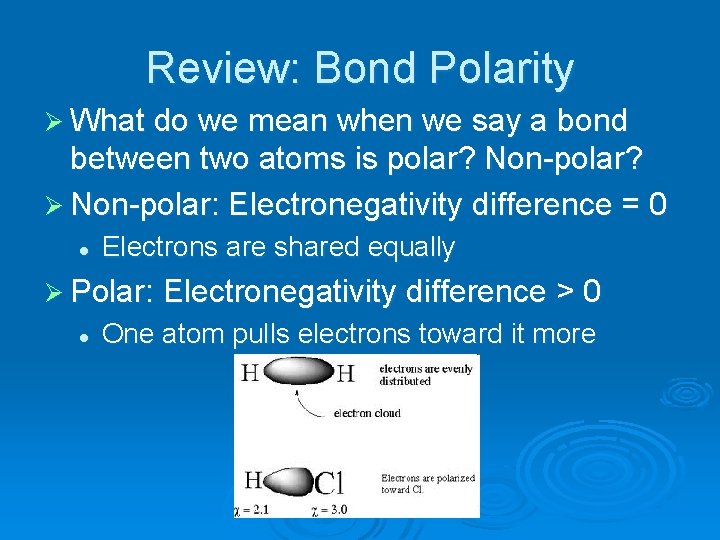
Review: Bond Polarity Ø What do we mean when we say a bond between two atoms is polar? Non-polar? Ø Non-polar: Electronegativity difference = 0 l Electrons are shared equally Ø Polar: Electronegativity difference > 0 l One atom pulls electrons toward it more

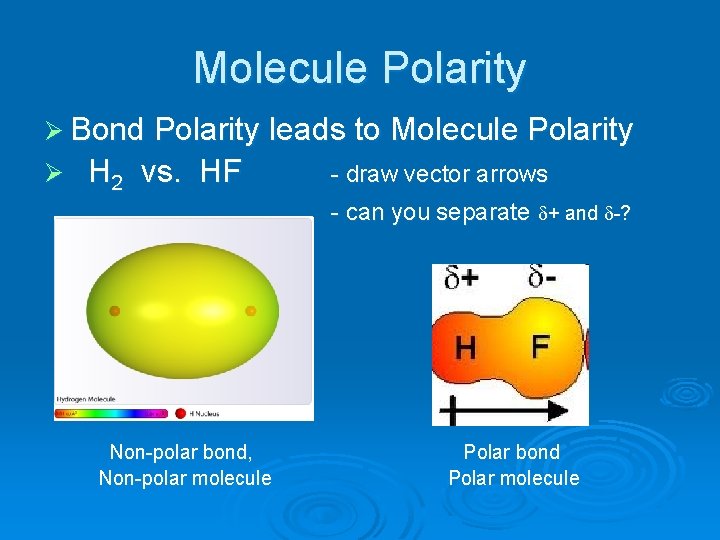
Molecule Polarity Ø Bond Polarity leads to Molecule Polarity Ø H 2 vs. HF - draw vector arrows - can you separate + and -? Non-polar bond, Non-polar molecule Polar bond Polar molecule

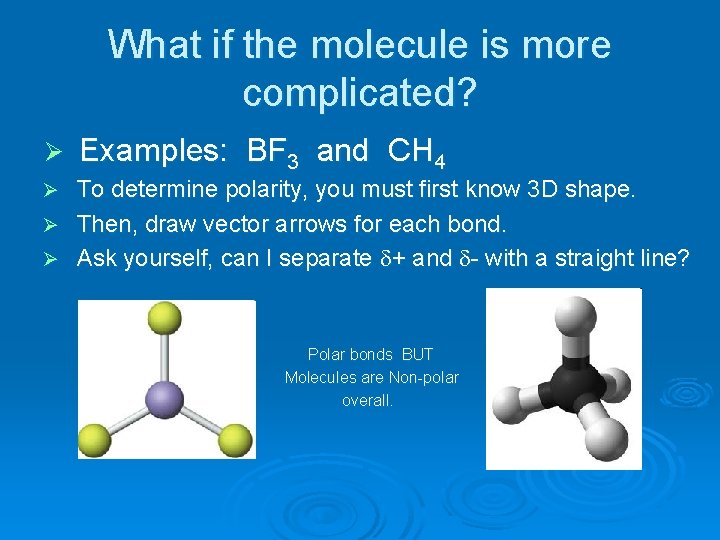
What if the molecule is more complicated? Ø Examples: BF 3 and CH 4 To determine polarity, you must first know 3 D shape. Ø Then, draw vector arrows for each bond. Ø Ask yourself, can I separate + and - with a straight line? Ø Polar bonds BUT Molecules are Non-polar overall.

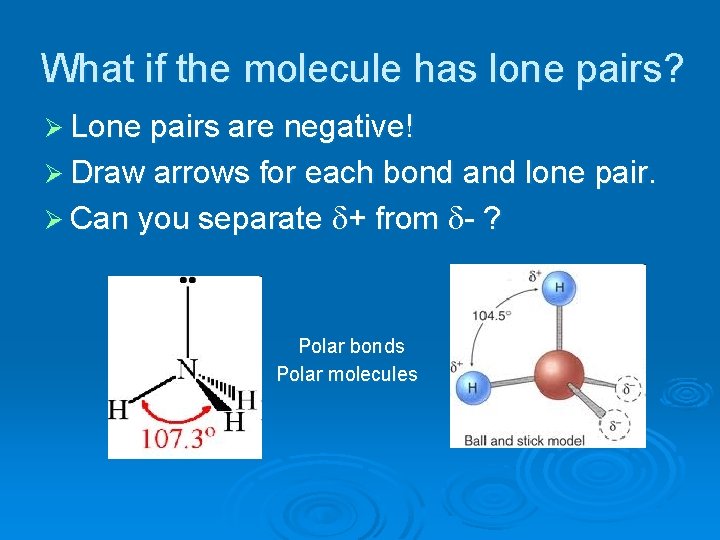
What if the molecule has lone pairs? Ø Lone pairs are negative! Ø Draw arrows for each bond and lone pair. Ø Can you separate + from - ? Polar bonds Polar molecules

Polar or Non-Polar? Ø Ø Ø Ø Si. F 4 l Non-polar molecule, polar bonds Al. Cl 3 l Non-polar molecule, polar bonds H 2 S l Polar molecule, polar bonds Cl 2 l Non-polar molecule, non-polar bond CS 2 l Non-polar molecule, polar bonds CBr 4 l Non-polar molecule, polar bonds PH 3 l Polar molecule, polar bonds

Summary Ø So… It is possible for molecules to have: l l l Non-polar bonds and be non-polar overall Polar bonds and be polar overall Ø Can there be molecules with non-polar bonds that are polar overall?

Intermolecular Forces Ø Intra vs. Inter Ø Attractions within a molecule (Intra) vs. Attractions between molecules (Inter). Ø Not as strong as chemical bonds. Ø Also play a role in macroscopic properties we can observe.

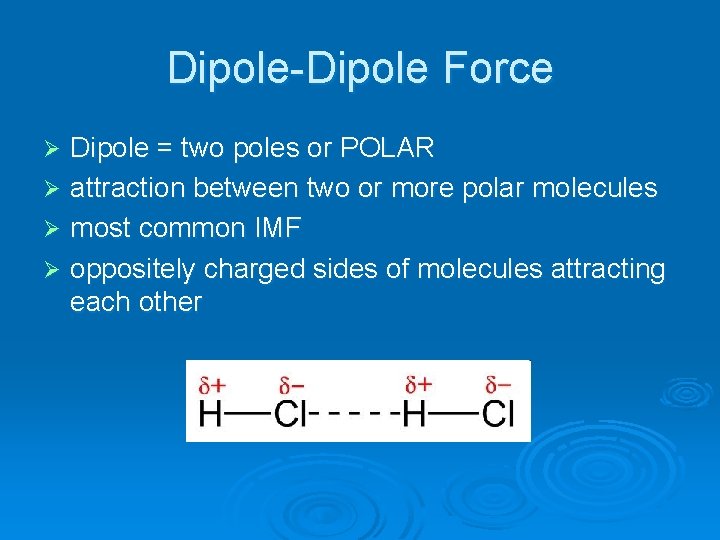
Dipole-Dipole Force Dipole = two poles or POLAR Ø attraction between two or more polar molecules Ø most common IMF Ø oppositely charged sides of molecules attracting each other Ø

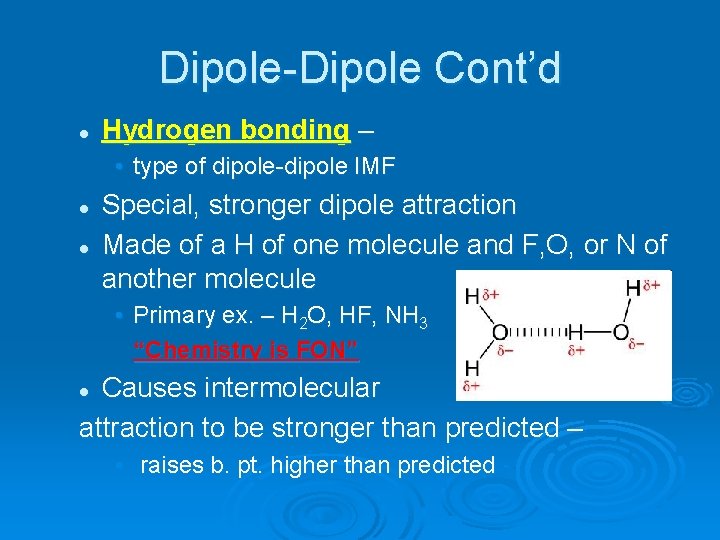
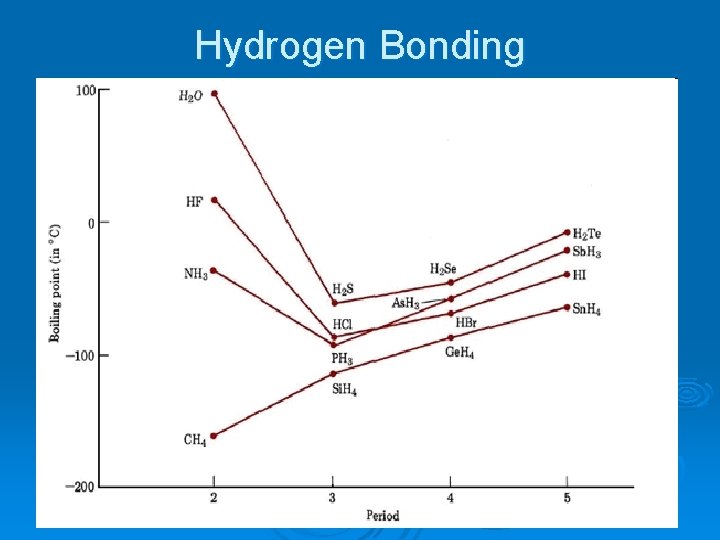
Dipole-Dipole Cont’d l Hydrogen bonding – • type of dipole-dipole IMF l l Special, stronger dipole attraction Made of a H of one molecule and F, O, or N of another molecule • Primary ex. – H 2 O, HF, NH 3 “Chemistry is FON” Causes intermolecular attraction to be stronger than predicted – l • raises b. pt. higher than predicted

Hydrogen Bonding

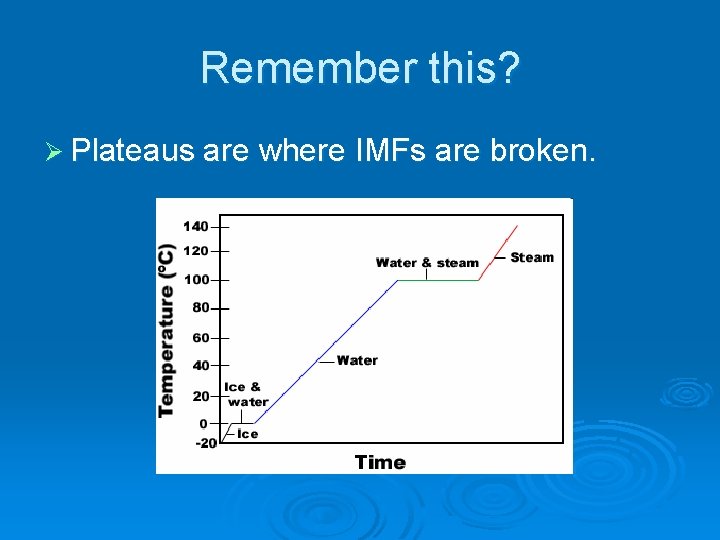
Remember this? Ø Plateaus are where IMFs are broken.

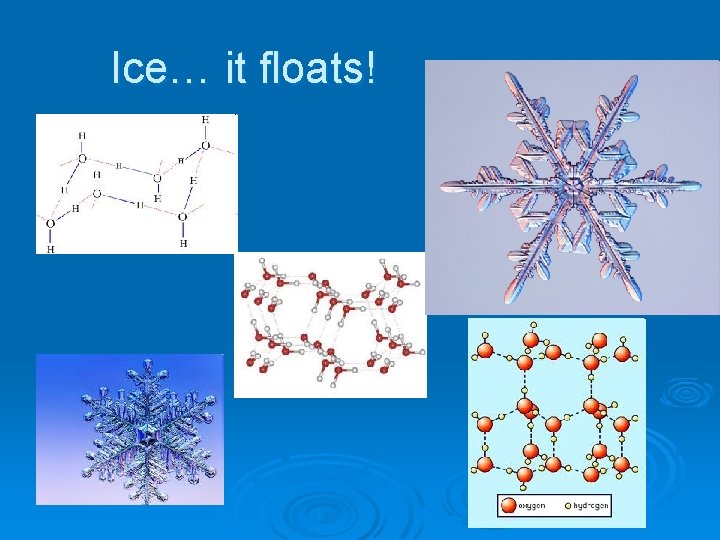
Ice… it floats!

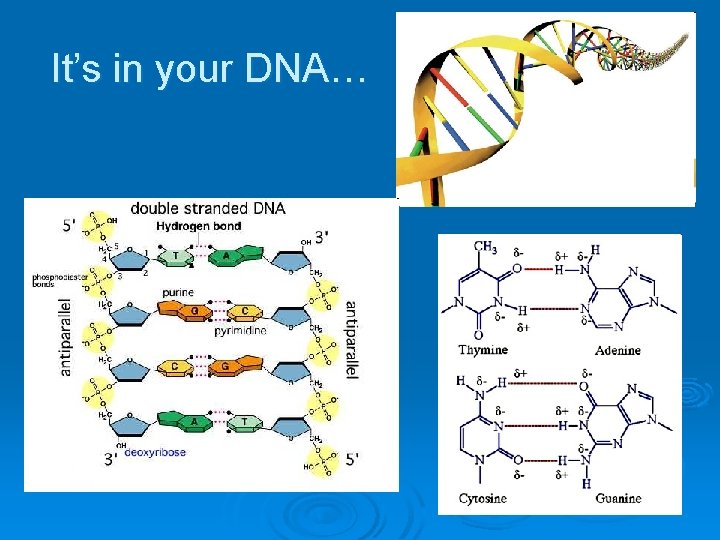
It’s in your DNA…

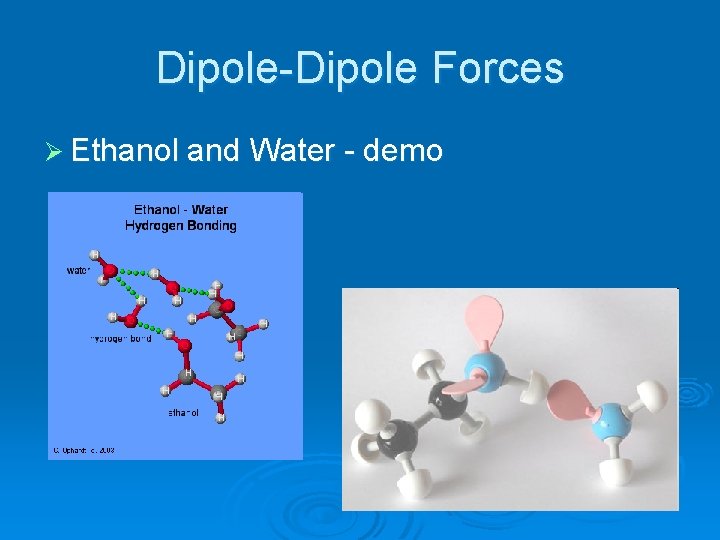
Dipole-Dipole Forces Ø Ethanol and Water - demo

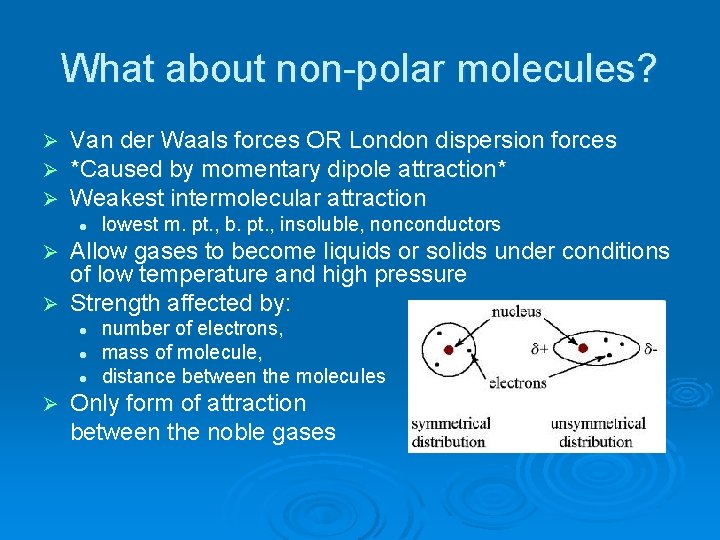
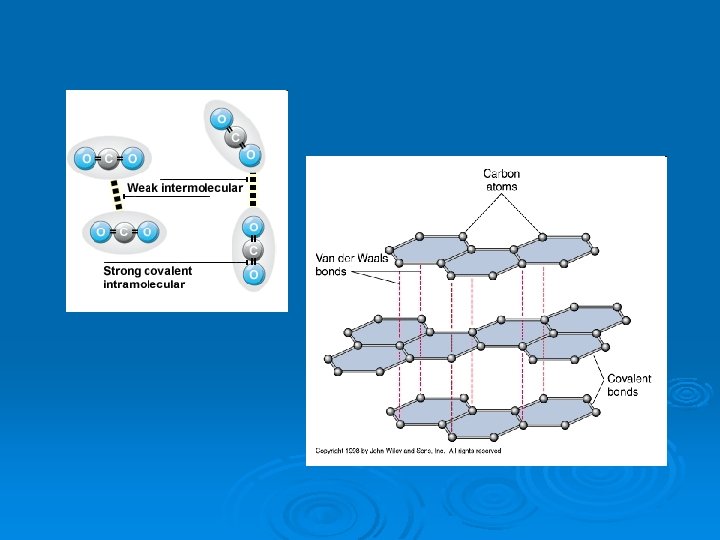
What about non-polar molecules? Ø Ø Ø Van der Waals forces OR London dispersion forces *Caused by momentary dipole attraction* Weakest intermolecular attraction l lowest m. pt. , b. pt. , insoluble, nonconductors Allow gases to become liquids or solids under conditions of low temperature and high pressure Ø Strength affected by: Ø l l l Ø number of electrons, mass of molecule, distance between the molecules Only form of attraction between the noble gases

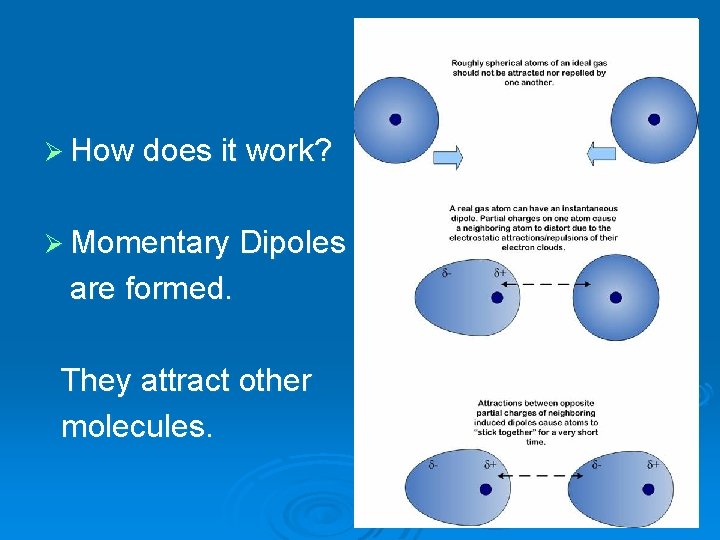
Ø How does it work? Ø Momentary Dipoles are formed. They attract other molecules.


Dry Ice Ø Solid CO 2 Ø Sublimation Ø http: //www. youtube. com/watch? v=p. P_l. Za Och. E 0

Molecule-Ion Attraction between ions and molecules Ø Most common when ionic solids dissolve in water Ø (+) ion will be attracted by negative side of water molecules and Ø (-) ion will be surrounded by the positive side of water molecule Ø Allows for electrical conductivity of solutions – mobile ions: increase number of ions, increase conductivity Ø

Ø Solutions of ionic compounds Video
 Is wax paper polar
Is wax paper polar Nonpolar polar and ionic bonds
Nonpolar polar and ionic bonds Quizlet
Quizlet Usp solubility
Usp solubility Fcl polar or nonpolar
Fcl polar or nonpolar H2o polar or nonpolar
H2o polar or nonpolar What makes polar bonds
What makes polar bonds Kondensator adalah
Kondensator adalah Nonpolar polar and ionic bonds
Nonpolar polar and ionic bonds Colloids shampoo uses
Colloids shampoo uses Cocaine lewis dot structure
Cocaine lewis dot structure Cocaine lewis dot structure
Cocaine lewis dot structure Covalent bond model
Covalent bond model Is hcn polar or nonpolar
Is hcn polar or nonpolar Types of molecular geometry
Types of molecular geometry Amino acid r groups
Amino acid r groups H-n-h-h polar or nonpolar
H-n-h-h polar or nonpolar Fno polar or nonpolar
Fno polar or nonpolar Oso polar or nonpolar
Oso polar or nonpolar Electronegativity and ph
Electronegativity and ph Molecular shape and polarity worksheet
Molecular shape and polarity worksheet Scl6 polar or nonpolar
Scl6 polar or nonpolar