Photometry Photometry means the measurement of light If









- Slides: 28


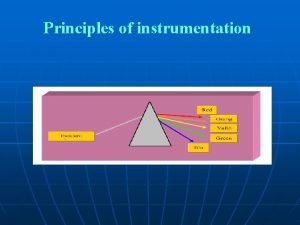
Photometry • Photometry means “the measurement of light” • If a substance can be converted to a soluble, colored material, its concentration may be determined by the amount of color present in the solution. • Photometer & Spectrophotometer are instruments used for this type of measutment, in which a photocell is used to detect the amount of light that passes through a colored solution from a light source.

Characteristics of Light • • Light is a form of electromagnetic energy that travels in waves. • Objects that appear colored absorb light at particular. The wavelength of light is the distance between two beaks of the light wave, it is inversely proportional with its energy.

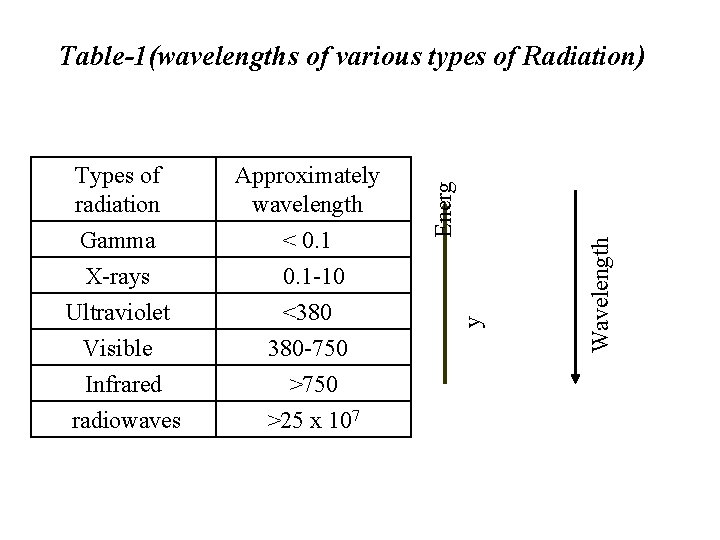
Gamma X-rays < 0. 1 -10 Ultraviolet Visible Infrared radiowaves <380 380 -750 >25 x 107 Wavelength Approximately wavelength y Types of radiation Energ Table-1(wavelengths of various types of Radiation)

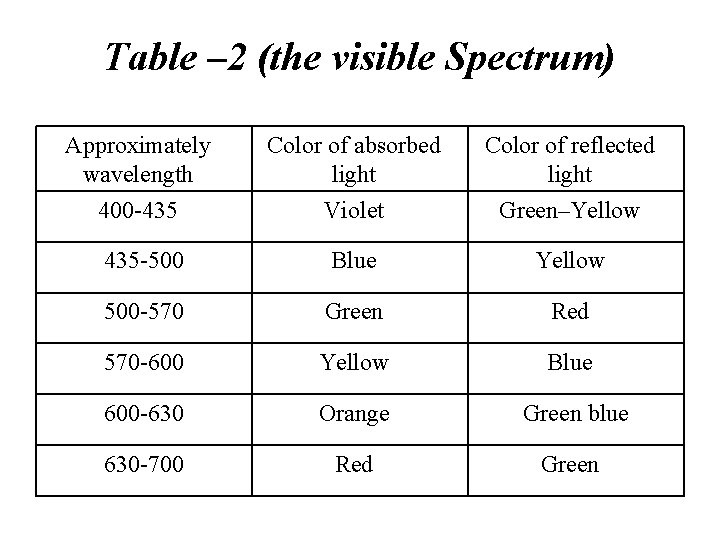
Table – 2 (the visible Spectrum) Approximately wavelength Color of absorbed light Color of reflected light 400 -435 Violet Green–Yellow 435 -500 Blue Yellow 500 -570 Green Red 570 -600 Yellow Blue 600 -630 Orange Green blue 630 -700 Red Green

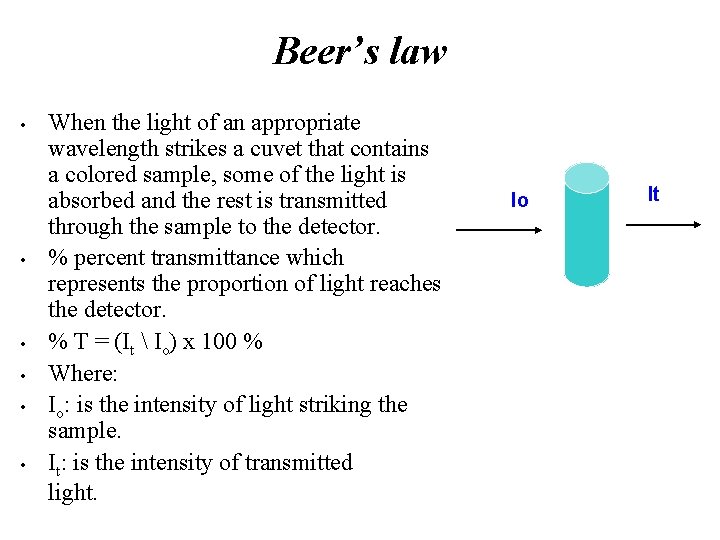
Beer’s law • • • When the light of an appropriate wavelength strikes a cuvet that contains a colored sample, some of the light is absorbed and the rest is transmitted through the sample to the detector. % percent transmittance which represents the proportion of light reaches the detector. % T = (It Io) x 100 % Where: Io: is the intensity of light striking the sample. It: is the intensity of transmitted light. Io It

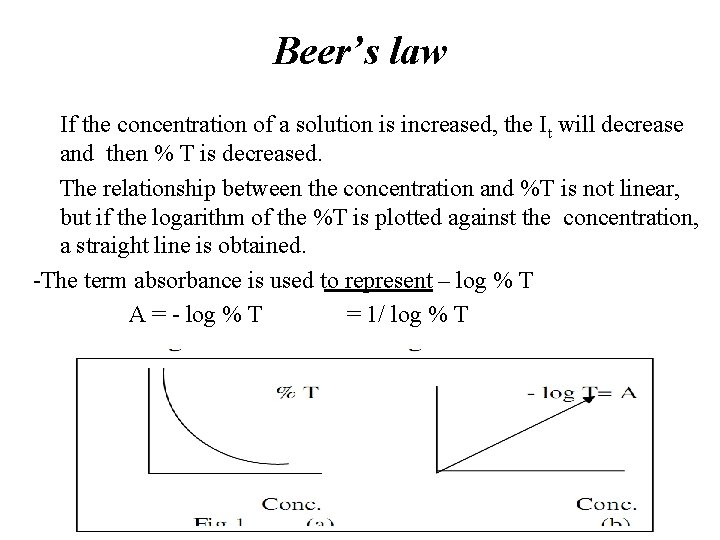
Beer’s law If the concentration of a solution is increased, the It will decrease and then % T is decreased. • The relationship between the concentration and %T is not linear, but if the logarithm of the %T is plotted against the concentration, a straight line is obtained. -The term absorbance is used to represent – log % T A = - log % T = 1/ log % T •

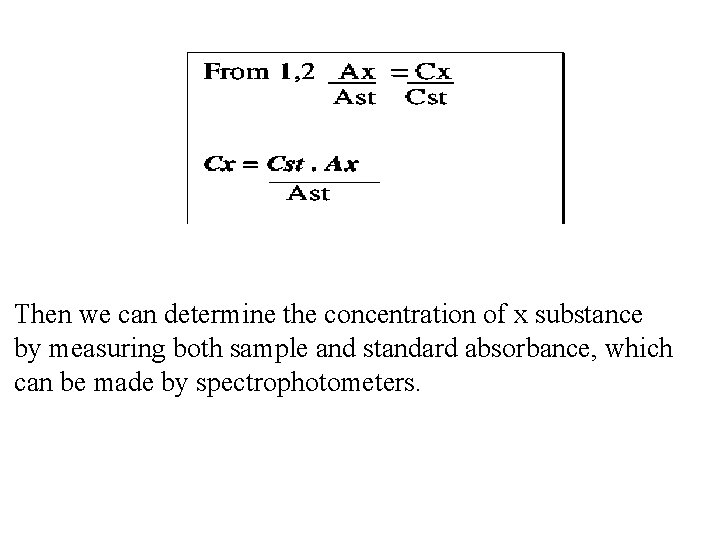
Then we can determine the concentration of x substance by measuring both sample and standard absorbance, which can be made by spectrophotometers.

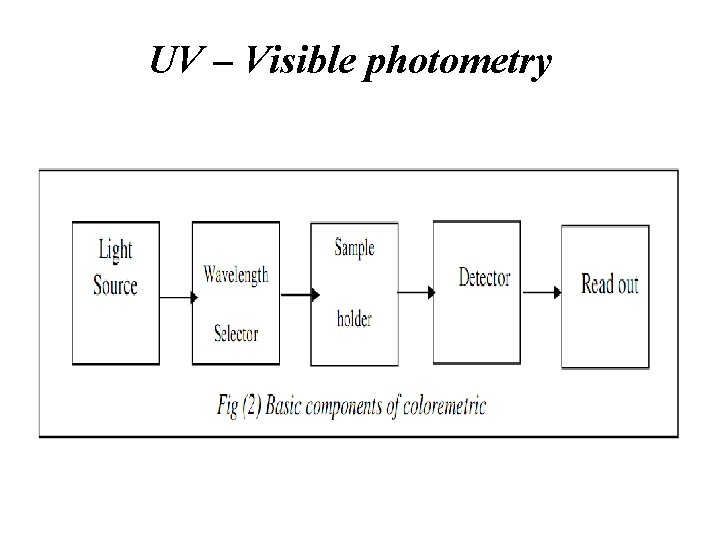
UV – Visible photometry • 1. 2. 3. 4. 5. Typical coloremetric instruments contain five components: Stable source of radiation energy. A transparent container for holding the sample. A device that isolates a restricted region of the spectrum for measurement. A radiation detector which converts radiant energy to electrical signals. A signal processor and read out which displays the transudated signals, a meter scale, a digital meter or a recorder chart.

UV – Visible photometry

Radiation sources - In UV region: The most commonly used is deuterium lamp or hydrogen lamp. In which a continues spectrum is produced by the excitation of deuterium (D 2) or hydrogen at law pressure, and then produced light with (160 -375) nm. - In visible region: Tungeston filament lamp is the most commonly used and produces light at (350 -2500) nm.

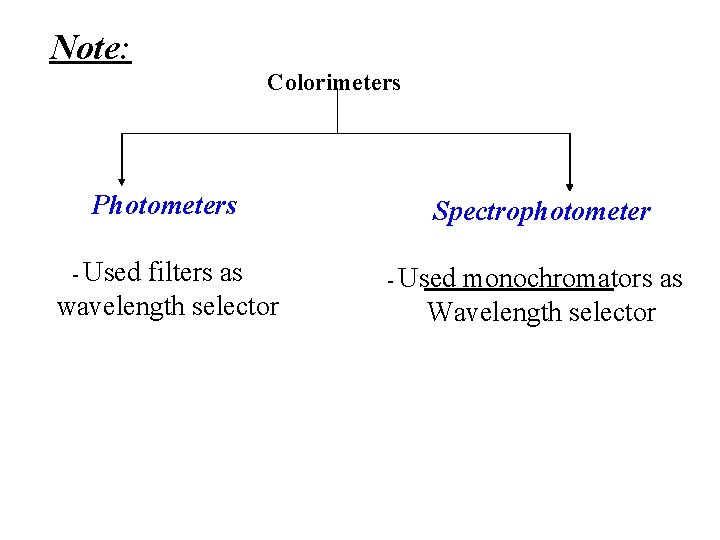
Note: Colorimeters Photometers - Used filters as wavelength selector Spectrophotometer - Used monochromators as Wavelength selector

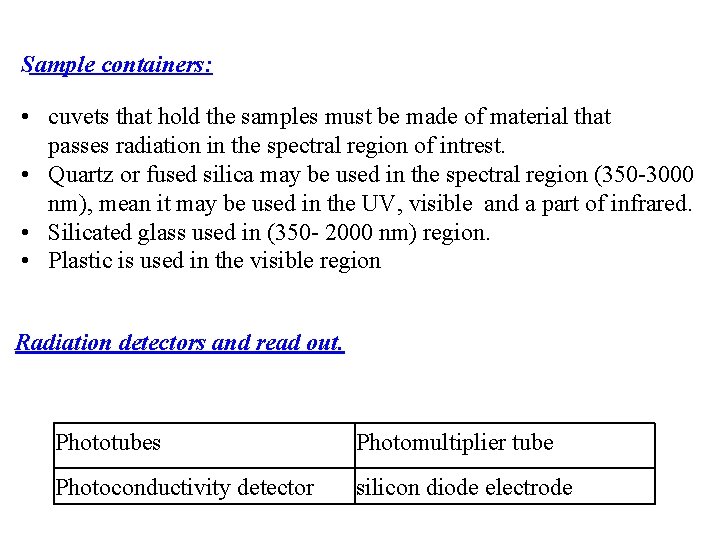
Sample containers: • cuvets that hold the samples must be made of material that passes radiation in the spectral region of intrest. • Quartz or fused silica may be used in the spectral region (350 -3000 nm), mean it may be used in the UV, visible and a part of infrared. • Silicated glass used in (350 - 2000 nm) region. • Plastic is used in the visible region Radiation detectors and read out. Phototubes Photomultiplier tube Photoconductivity detector silicon diode electrode


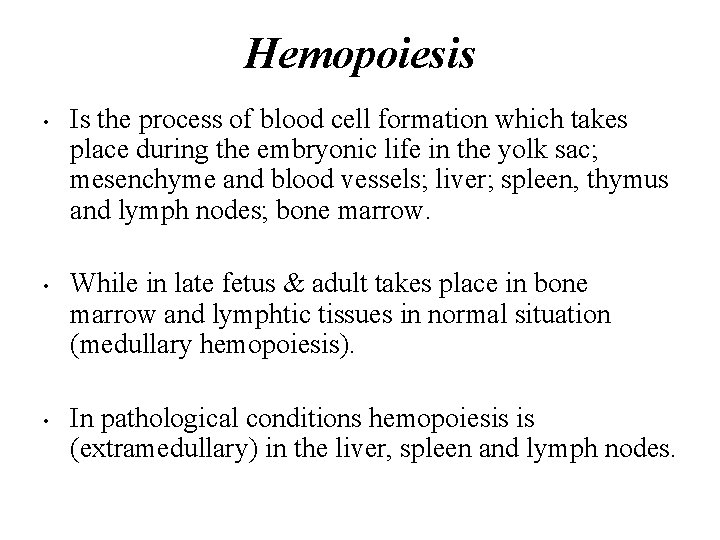
Hemopoiesis • • • Is the process of blood cell formation which takes place during the embryonic life in the yolk sac; mesenchyme and blood vessels; liver; spleen, thymus and lymph nodes; bone marrow. While in late fetus & adult takes place in bone marrow and lymphtic tissues in normal situation (medullary hemopoiesis). In pathological conditions hemopoiesis is (extramedullary) in the liver, spleen and lymph nodes.

• • • Hemoglobin (Hb) is the standard abbreviation for hemoglobin, the oxygen-carrying pigment and predominant protein in the red blood cells. Hemoglobin is the protein that carries oxygen from the lungs to the tissues and carries carbon dioxide from the tissues back to the lungs. In order to function most efficiently, hemoglobin needs to bind to oxygen tightly in the oxygen-rich atmosphere of the lungs and be able to release oxygen rapidly in the relatively oxygen-poor environment of the tissues.

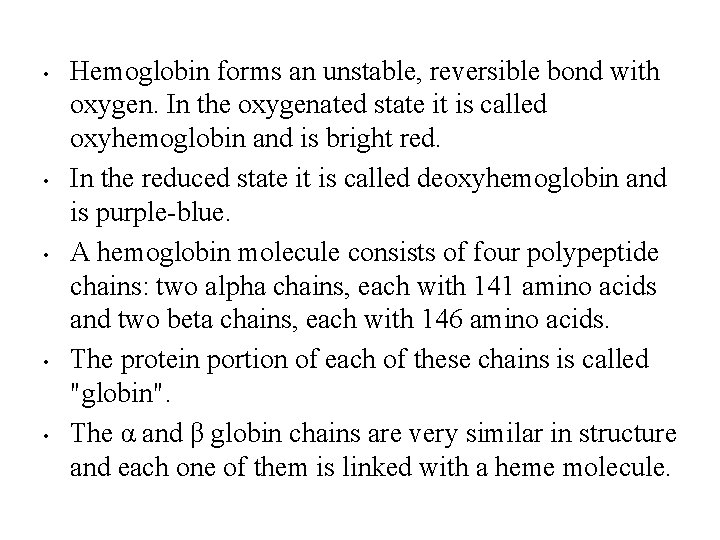
• • • Hemoglobin forms an unstable, reversible bond with oxygen. In the oxygenated state it is called oxyhemoglobin and is bright red. In the reduced state it is called deoxyhemoglobin and is purple-blue. A hemoglobin molecule consists of four polypeptide chains: two alpha chains, each with 141 amino acids and two beta chains, each with 146 amino acids. The protein portion of each of these chains is called "globin". The α and β globin chains are very similar in structure and each one of them is linked with a heme molecule.

• • A heme group is a flat ring molecule containing carbon, nitrogen and hydrogen atoms, with a single Fe 2+ ion at the center. Without the iron, the ring is called a porphyrin. Changes in the amino acid sequence of these chains results in abnormal hemoglobin's. For example, hemoglobin S is found in sickle-cell disease, a severe type of anemia in which the red cells become sickle-shaped when oxygen is in short supply.


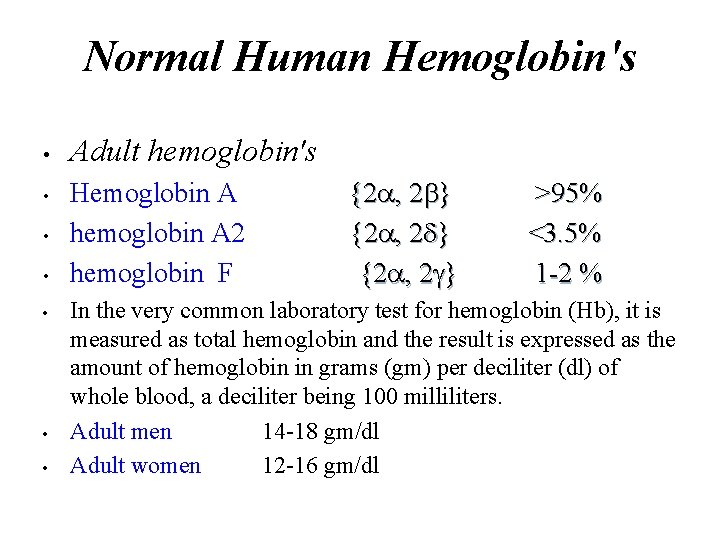
Normal Human Hemoglobin's • • Adult hemoglobin's Hemoglobin A hemoglobin A 2 hemoglobin F {2 , 2 } >95% <3. 5% 1 -2 % In the very common laboratory test for hemoglobin (Hb), it is measured as total hemoglobin and the result is expressed as the amount of hemoglobin in grams (gm) per deciliter (dl) of whole blood, a deciliter being 100 milliliters. Adult men 14 -18 gm/dl Adult women 12 -16 gm/dl

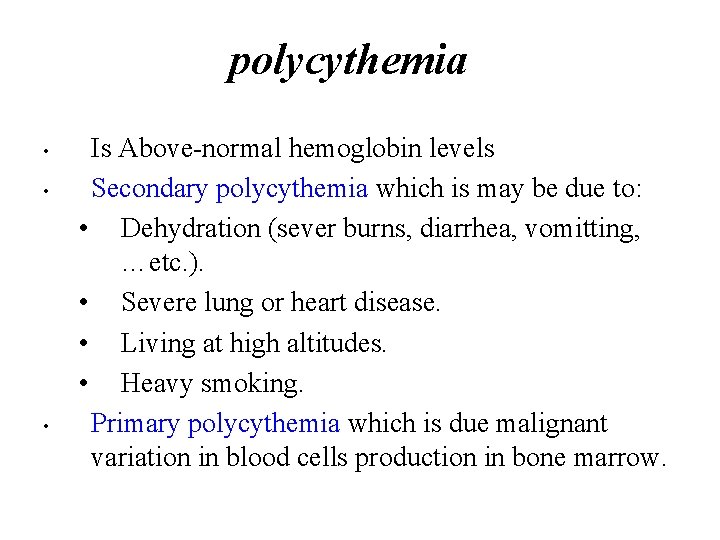
polycythemia • • • Is Above-normal hemoglobin levels Secondary polycythemia which is may be due to: • Dehydration (sever burns, diarrhea, vomitting, …etc. ). • Severe lung or heart disease. • Living at high altitudes. • Heavy smoking. Primary polycythemia which is due malignant variation in blood cells production in bone marrow.

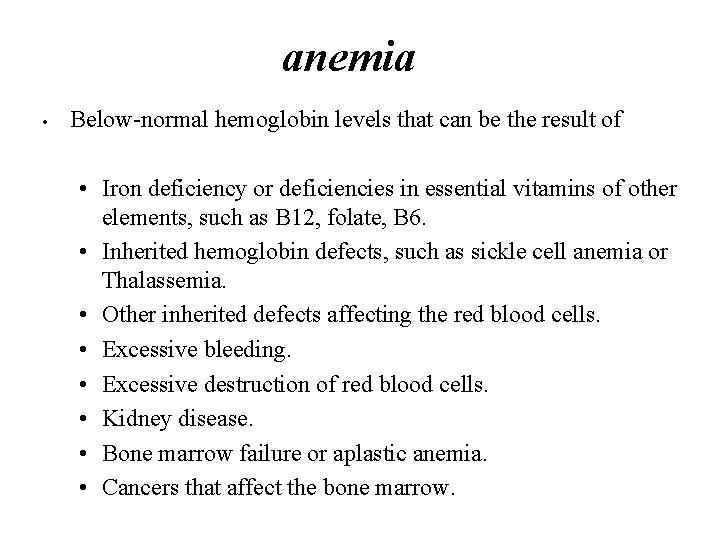
anemia • Below-normal hemoglobin levels that can be the result of • Iron deficiency or deficiencies in essential vitamins of other elements, such as B 12, folate, B 6. • Inherited hemoglobin defects, such as sickle cell anemia or Thalassemia. • Other inherited defects affecting the red blood cells. • Excessive bleeding. • Excessive destruction of red blood cells. • Kidney disease. • Bone marrow failure or aplastic anemia. • Cancers that affect the bone marrow.

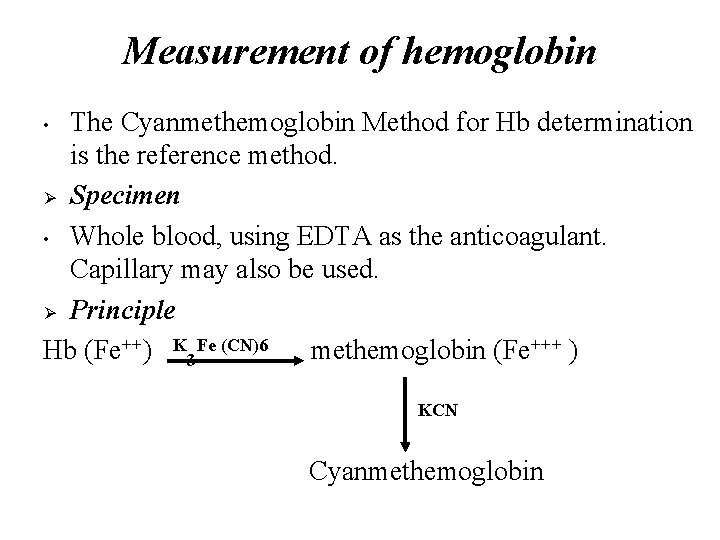
Measurement of hemoglobin The Cyanmethemoglobin Method for Hb determination is the reference method. Ø Specimen • Whole blood, using EDTA as the anticoagulant. Capillary may also be used. Ø Principle Hb (Fe++) K 3 Fe (CN)6 methemoglobin (Fe+++ ) • KCN Cyanmethemoglobin

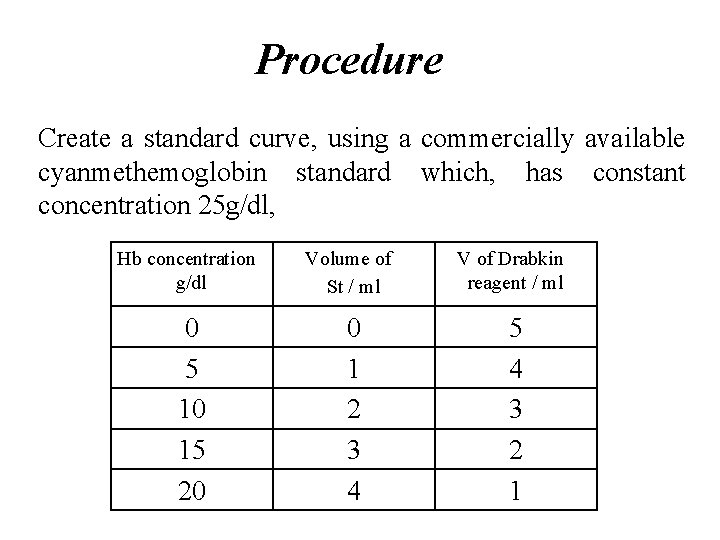
Procedure Create a standard curve, using a commercially available cyanmethemoglobin standard which, has constant concentration 25 g/dl, Hb concentration g/dl Volume of St / ml V of Drabkin reagent / ml 0 5 10 15 20 0 1 2 3 4 5 4 3 2 1

Procedure • • • Put 5 ml of pre-prepared working reagent in a test tube. Then add 20 µL blood to the test tube. Mix well and leave it at room temp. for 3 -5 minutes. Measure the absorbance for cyanmethemoglobin at wave length 540 nm against reagent blank.

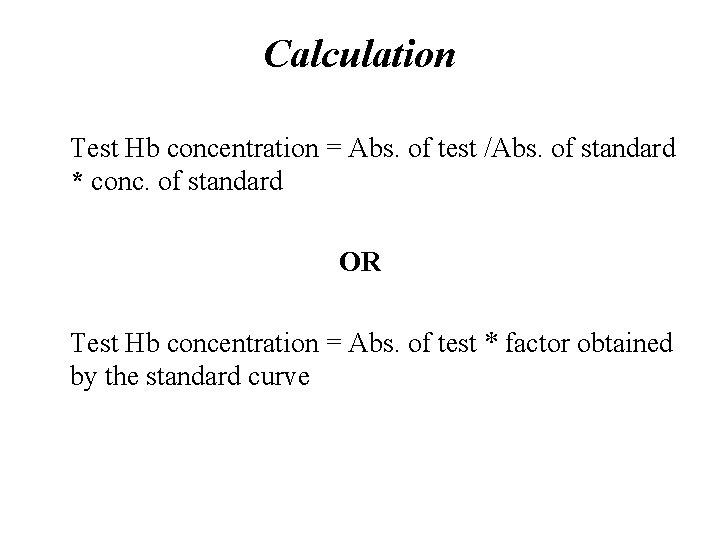
Calculation • Test Hb concentration = Abs. of test /Abs. of standard * conc. of standard OR • Test Hb concentration = Abs. of test * factor obtained by the standard curve

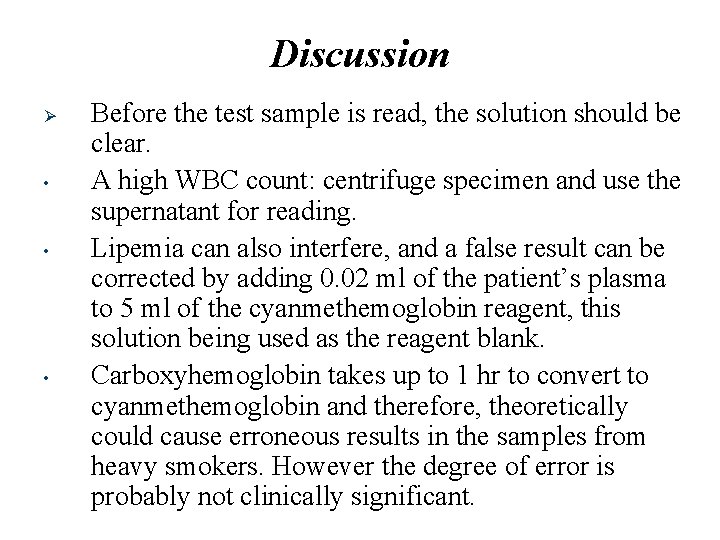
Discussion Ø • • • Before the test sample is read, the solution should be clear. A high WBC count: centrifuge specimen and use the supernatant for reading. Lipemia can also interfere, and a false result can be corrected by adding 0. 02 ml of the patient’s plasma to 5 ml of the cyanmethemoglobin reagent, this solution being used as the reagent blank. Carboxyhemoglobin takes up to 1 hr to convert to cyanmethemoglobin and therefore, theoretically could cause erroneous results in the samples from heavy smokers. However the degree of error is probably not clinically significant.

 Light light light chapter 23
Light light light chapter 23 Into the light chapter 22
Into the light chapter 22 Light light light chapter 22
Light light light chapter 22 What does ø mean in trigonometry
What does ø mean in trigonometry White light measurement
White light measurement Goniometer light measurement
Goniometer light measurement Coloremetric
Coloremetric Flame photometer introduction
Flame photometer introduction Beer's law
Beer's law Photometry
Photometry Reflectance photometry principle
Reflectance photometry principle Iraf imexamine
Iraf imexamine Photometry
Photometry Photometry
Photometry Poly means many and gon means
Poly means many and gon means Metamorphic rocks
Metamorphic rocks Meta means change and morph means heat
Meta means change and morph means heat Biodiversity and conservation
Biodiversity and conservation Bio means life logy means
Bio means life logy means Put out the light and put out the light
Put out the light and put out the light Bacteria double membrane
Bacteria double membrane The bouncing off of light.
The bouncing off of light. Block light in science
Block light in science Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton