PHOTODEGRADATON OF WATERORGANC CONTAMNANTS WTH SAFE SENSTZED Zn









- Slides: 23

PHOTODEGRADATİON OF WATER-ORGANİC CONTAMİNANTS WİTH SAFE SENSİTİZED Zn. O NANOPARTİCLES: A GREEN SUSTAİNABLE METHOD FOR WATER PURİFİCATİON Presented by: Amani Zu’bi Coauthors: Ahed Zyoud & Hikmat Hilal ICAME-2017 Yildiz University Chemistry Department / An-Najah N. University, Palestien 1 1

• • • The problem to solve Palestine suffers serious shortages in pure water supplies. Due to the abuse of ground water resources practiced by settlers. They also dispose their sewage randomly into Palestinian areas Most commonly: (Industrial dyes, phenolderivatives herbicides, pesticides, insecticides, drugs … ec) and heavy metals. Contaminants reach ground & surface water Severe pollution occurs. Purification & recycling of water is needed. 2 ICAME-2017 Yildiz University

• Different types strategies are used to remove contaminants. But expensive or not efficient. • Recently: Natural solar energy has been examined for complete mineralization. • Core issue is developing light-sensitive semiconducting materials to catalyze photodegradation 3 ICAME-2017 Yildiz University

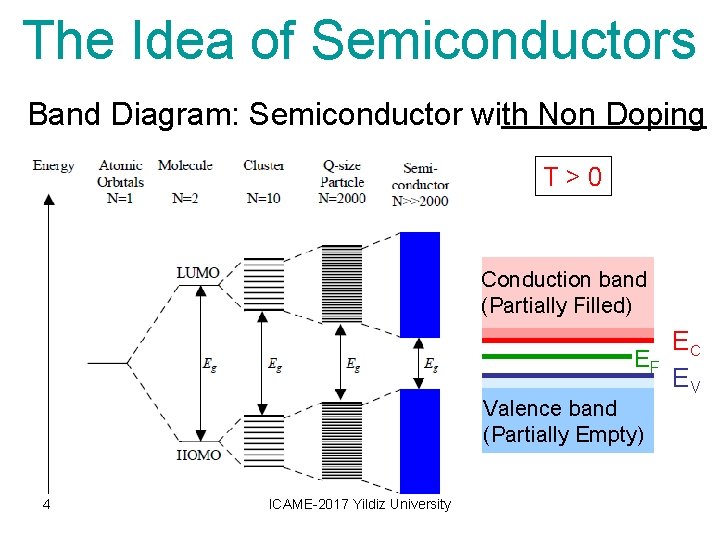
The Idea of Semiconductors Band Diagram: Semiconductor with Non Doping T>0 Conduction band (Partially Filled) EC EF EV Valence band (Partially Empty) 4 ICAME-2017 Yildiz University

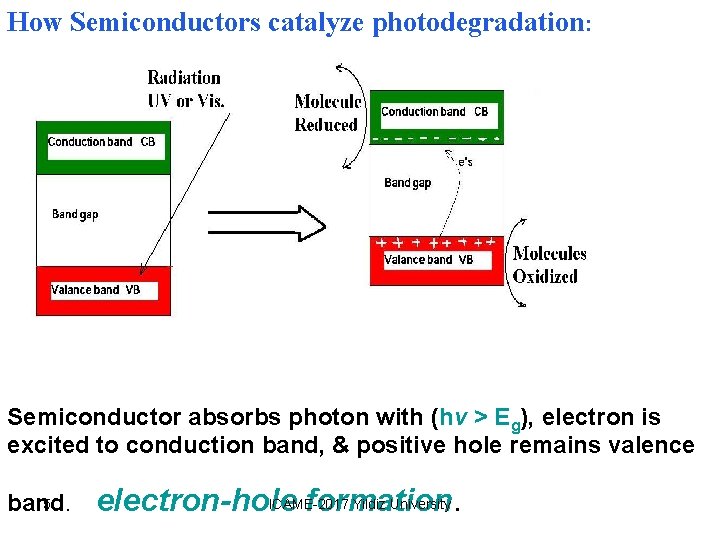
How Semiconductors catalyze photodegradation: Semiconductor absorbs photon with (hν > Eg), electron is excited to conduction band, & positive hole remains valence 5 band. ICAME-2017 Yildiz University electron-hole formation.

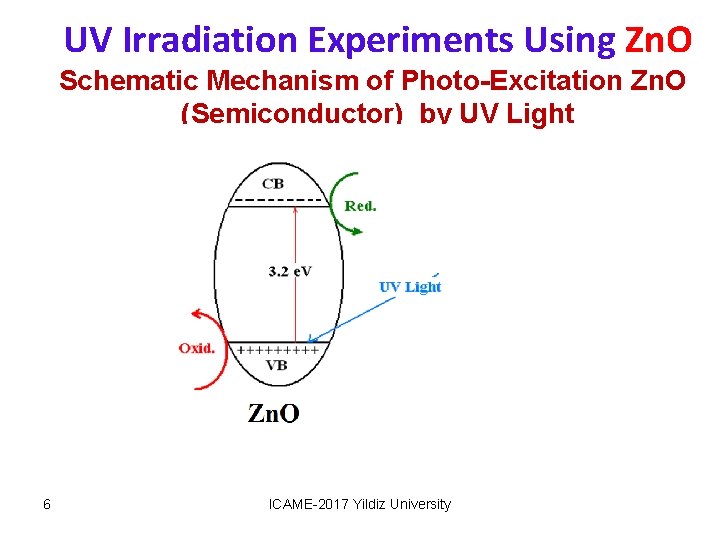
UV Irradiation Experiments Using Zn. O Schematic Mechanism of Photo-Excitation Zn. O (Semiconductor) by UV Light 6 ICAME-2017 Yildiz University

The purpose: Using lab prepared Zn. O nano-powder and using it in degrading organic contaminants (Methyle Orange) under solar simulator light using the UV fraction (~4%), taking into account: 1 - efficiency 2 - recyclability 3 - cost 4 - environmental safety 5 - working under natural conditions. 7 ICAME-2017 Yildiz University

Zn. O Characterization 8 ICAME-2017 Yildiz University

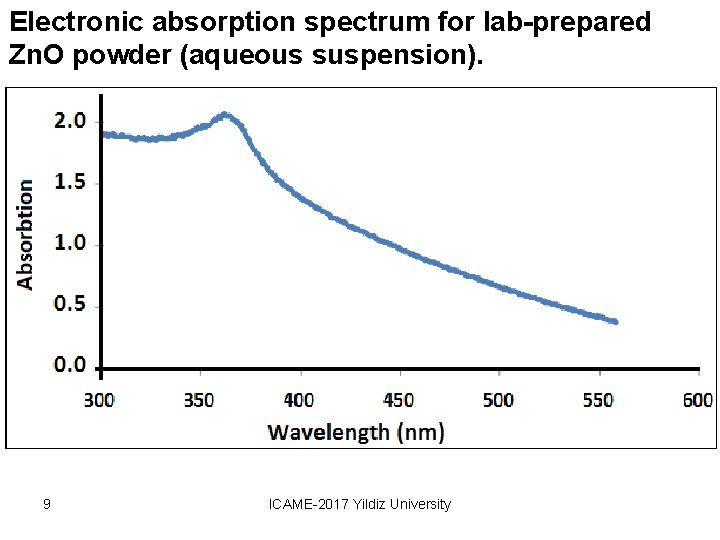
Electronic absorption spectrum for lab-prepared Zn. O powder (aqueous suspension). 9 ICAME-2017 Yildiz University

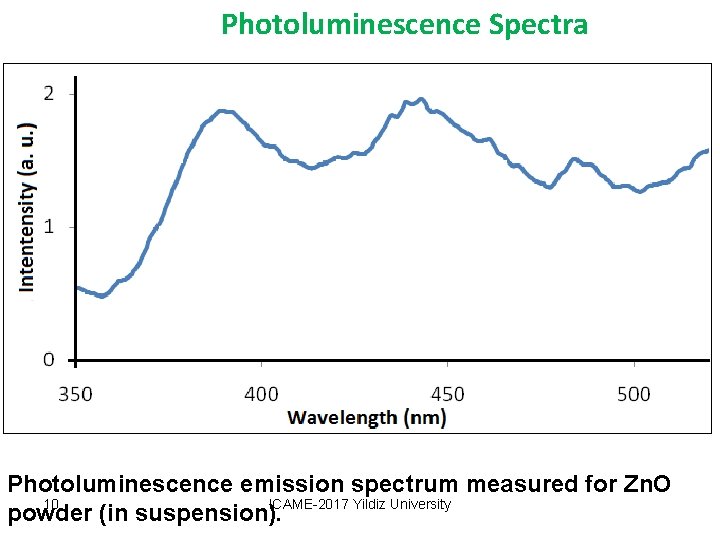
Photoluminescence Spectra Photoluminescence emission spectrum measured for Zn. O 10 ICAME-2017 Yildiz University powder (in suspension).

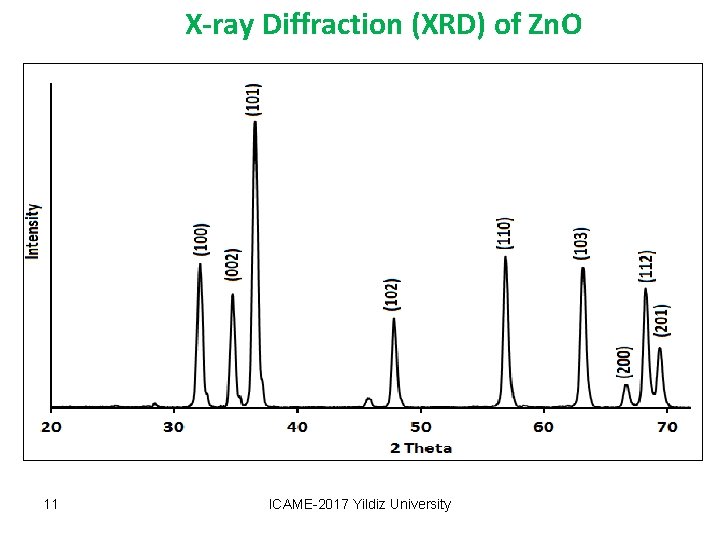
X-ray Diffraction (XRD) of Zn. O 11 ICAME-2017 Yildiz University

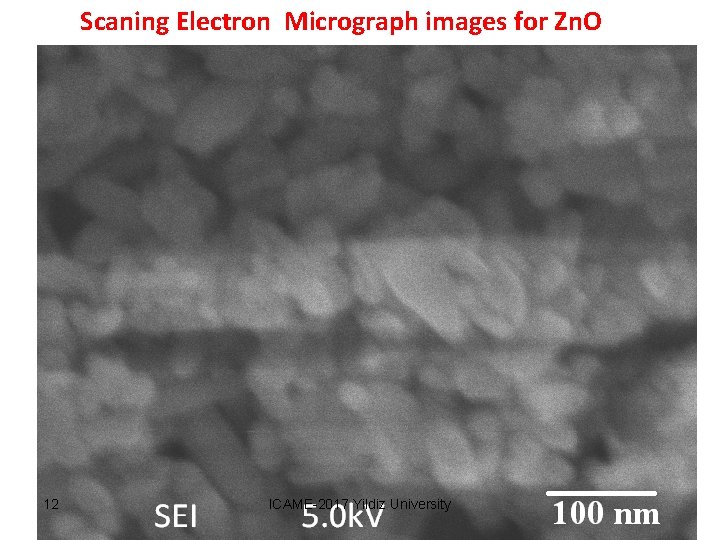
Scaning Electron Micrograph images for Zn. O 17 nm 12 ICAME-2017 Yildiz University

Solar Simulator Photodegradation Experiments Measuring The %degradation Turn over number Quantum yeild underdifferent Experiment conditions 13 ICAME-2017 Yildiz University

Methyl Orange 14 ICAME-2017 Yildiz University

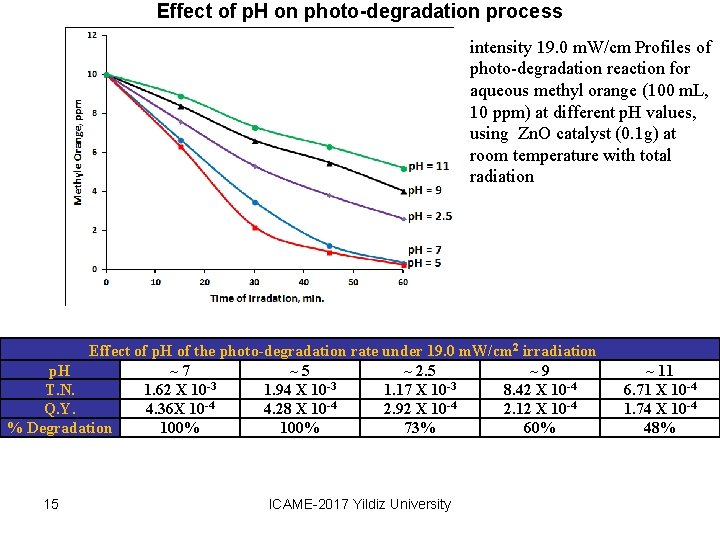
Effect of p. H on photo-degradation process intensity 19. 0 m. W/cm Profiles of photo-degradation reaction for aqueous methyl orange (100 m. L, 10 ppm) at different p. H values, using Zn. O catalyst (0. 1 g) at room temperature with total radiation Effect of p. H of the photo-degradation rate under 19. 0 m. W/cm 2 irradiation p. H ~ 7 ~ 5 ~ 2. 5 ~ 9 T. N. 1. 62 X 10 -3 1. 94 X 10 -3 1. 17 X 10 -3 8. 42 X 10 -4 Q. Y. 4. 36 X 10 -4 4. 28 X 10 -4 2. 92 X 10 -4 2. 12 X 10 -4 % Degradation 100% 73% 60% 15 ICAME-2017 Yildiz University ~ 11 6. 71 X 10 -4 1. 74 X 10 -4 48%

Effect of temperature Profiles of photodegradation reaction of methyl orange solution (100 m. L, 20 ppm) at different temperatures, using Zn. O catalyst (0. 1 g) and total irradiation intensity of 19. 0 m. W/cm 2 at p. H ~7 Temp. T. N. Q. Y. % Degradation 16 10 o. C 1. 86 X 10 -3 3. 52 X 10 -4 62% 20 o. C 2. 38 X 10 -3 4. 28 X 10 -4 70% ICAME-2017 Yildiz University 30 o. C 2. 87 X 10 -3 5. 44 X 10 -4 84% 45 o. C 2. 80 X 10 -3 5. 29 X 10 -4 81%

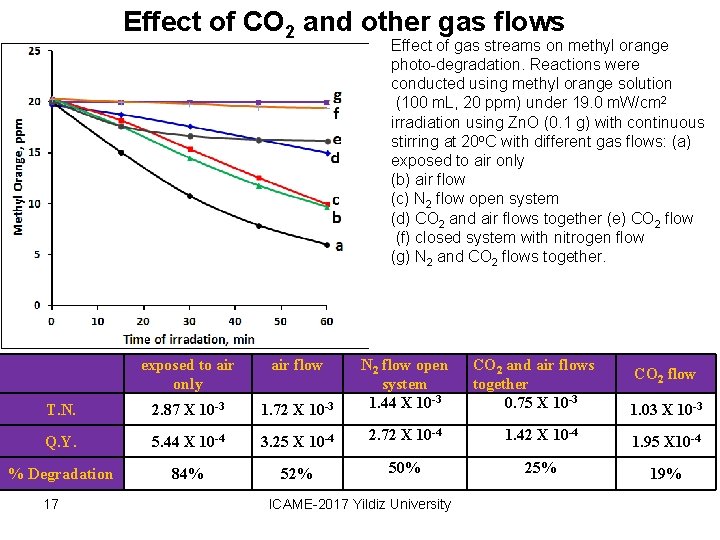
Effect of CO 2 and other gas flows Effect of gas streams on methyl orange photo-degradation. Reactions were conducted using methyl orange solution (100 m. L, 20 ppm) under 19. 0 m. W/cm 2 irradiation using Zn. O (0. 1 g) with continuous stirring at 20 o. C with different gas flows: (a) exposed to air only (b) air flow (c) N 2 flow open system (d) CO 2 and air flows together (e) CO 2 flow (f) closed system with nitrogen flow (g) N 2 and CO 2 flows together. exposed to air only air flow T. N. 2. 87 X 10 -3 1. 72 X 10 -3 N 2 flow open system 1. 44 X 10 -3 Q. Y. 5. 44 X 10 -4 3. 25 X 10 -4 2. 72 X 10 -4 1. 42 X 10 -4 1. 95 X 10 -4 % Degradation 84% 52% 50% 25% 19% 17 ICAME-2017 Yildiz University CO 2 and air flows together 0. 75 X 10 -3 CO 2 flow 1. 03 X 10 -3

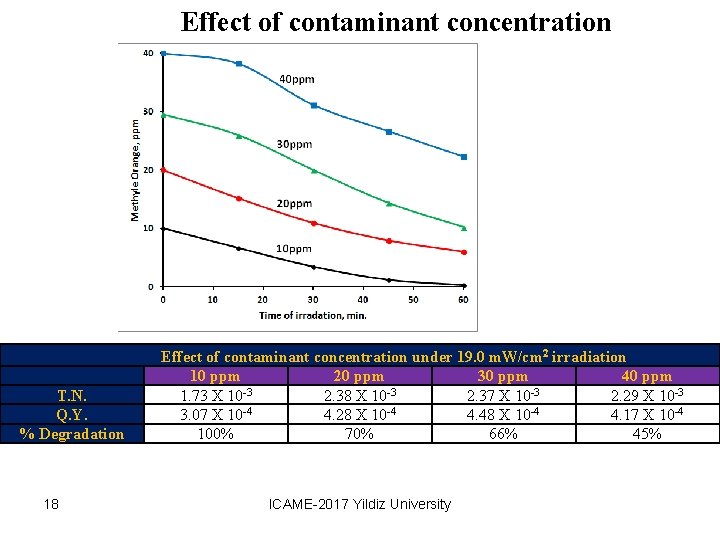
Effect of contaminant concentration Effect of contaminant concentration under 19. 0 m. W/cm 2 irradiation 10 ppm 20 ppm 30 ppm 40 ppm -3 -3 -3 T. N. 1. 73 X 10 2. 38 X 10 2. 37 X 10 2. 29 X 10 -3 Q. Y. 3. 07 X 10 -4 4. 28 X 10 -4 4. 48 X 10 -4 4. 17 X 10 -4 % Degradation 100% 70% 66% 45% 18 ICAME-2017 Yildiz University

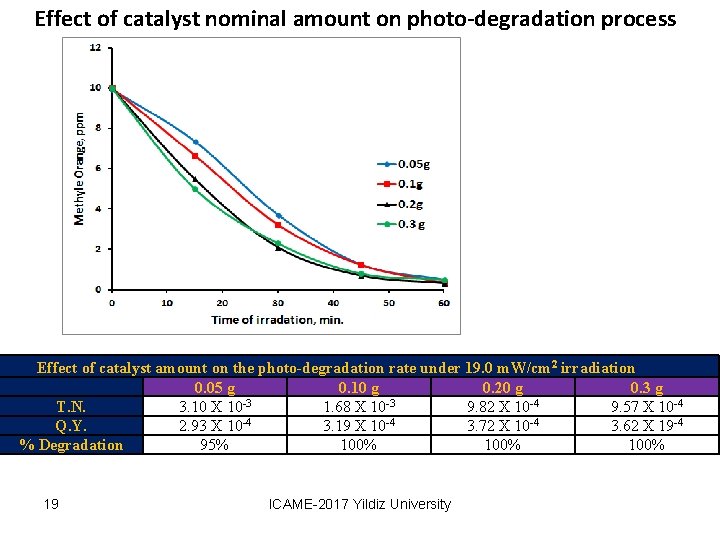
Effect of catalyst nominal amount on photo-degradation process Effect of catalyst amount on the photo-degradation rate under 19. 0 m. W/cm 2 irradiation 0. 05 g 0. 10 g 0. 20 g 0. 3 g -3 -3 -4 T. N. 3. 10 X 10 1. 68 X 10 9. 82 X 10 9. 57 X 10 -4 Q. Y. 2. 93 X 10 -4 3. 19 X 10 -4 3. 72 X 10 -4 3. 62 X 19 -4 % Degradation 95% 100% 19 ICAME-2017 Yildiz University

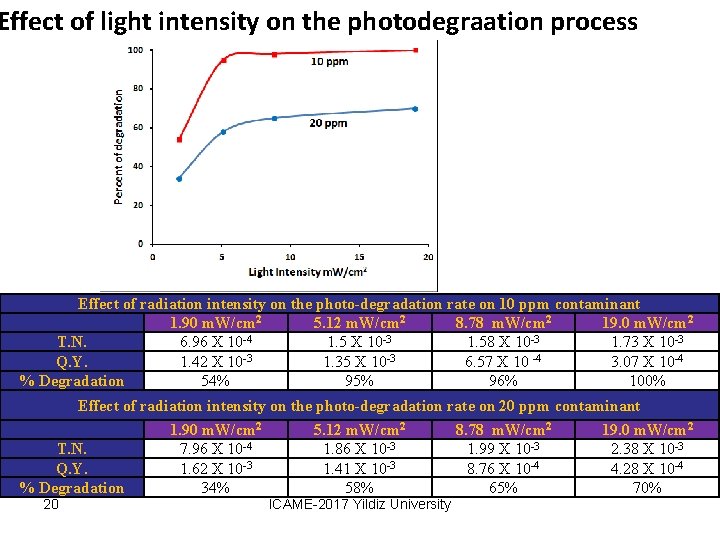
Effect of light intensity on the photodegraation process Effect of radiation intensity on the photo-degradation rate on 10 ppm contaminant 1. 90 m. W/cm 2 5. 12 m. W/cm 2 8. 78 m. W/cm 2 19. 0 m. W/cm 2 T. N. 6. 96 X 10 -4 1. 5 X 10 -3 1. 58 X 10 -3 1. 73 X 10 -3 Q. Y. 1. 42 X 10 -3 1. 35 X 10 -3 6. 57 X 10 -4 3. 07 X 10 -4 % Degradation 54% 95% 96% 100% Effect of radiation intensity on the photo-degradation rate on 20 ppm contaminant T. N. Q. Y. % Degradation 20 1. 90 m. W/cm 2 7. 96 X 10 -4 1. 62 X 10 -3 34% 5. 12 m. W/cm 2 1. 86 X 10 -3 1. 41 X 10 -3 58% ICAME-2017 Yildiz University 8. 78 m. W/cm 2 1. 99 X 10 -3 8. 76 X 10 -4 65% 19. 0 m. W/cm 2 2. 38 X 10 -3 4. 28 X 10 -4 70%

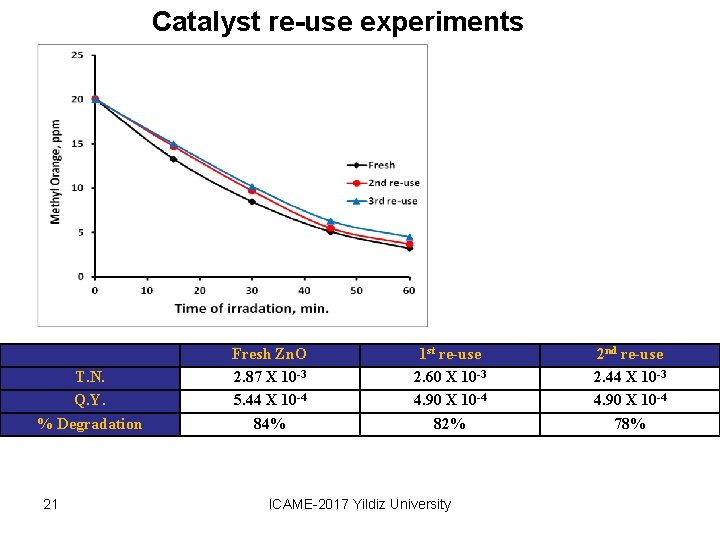
Catalyst re-use experiments T. N. Q. Y. % Degradation 21 Fresh Zn. O 2. 87 X 10 -3 5. 44 X 10 -4 84% 1 st re-use 2. 60 X 10 -3 4. 90 X 10 -4 82% ICAME-2017 Yildiz University 2 nd re-use 2. 44 X 10 -3 4. 90 X 10 -4 78%

Complete mineralization 22 ICAME-2017 Yildiz University

Thank You 23 ICAME-2017 Yildiz University
 Safe people safe places
Safe people safe places Safe feed safe food
Safe feed safe food Comparatives and superlatives exceptions
Comparatives and superlatives exceptions Is canoga park safe
Is canoga park safe Restraint alternatives and safe restraint use
Restraint alternatives and safe restraint use Unit 14 safe use of pesticides
Unit 14 safe use of pesticides Safe surgery saves lives
Safe surgery saves lives Center line font
Center line font Acetylene is unstable at pressures above ____ psig
Acetylene is unstable at pressures above ____ psig Chromophtal
Chromophtal Keeping an infant safe and well section 7-3
Keeping an infant safe and well section 7-3 Us powerboating safe powerboat handling
Us powerboating safe powerboat handling Fattom chart
Fattom chart Are you sure this is safe
Are you sure this is safe Safe surgery coalition
Safe surgery coalition Safe rigging practices
Safe rigging practices Safe and together model principles
Safe and together model principles Sissy safe browser
Sissy safe browser Open design security
Open design security Menstrual cycle chart
Menstrual cycle chart Is unchecky safe
Is unchecky safe Cwru respondus lockdown browser
Cwru respondus lockdown browser Safe beginnings
Safe beginnings Food storage principles
Food storage principles