FSC 36 Safe FeedSafe Food Guidance Document Version























- Slides: 29

FSC 36 Safe Feed/Safe Food: Guidance Document Version 7. 0 Training June 2017

FSC 36 Safe Feed/Safe Food Training AGENDA • Very Quick Review Food Safety Modernization Act • Guidance Document: Old vs. New • Questions Please ask Questions as we cover the information. GOAL: address your questions and help you understand the version 7. 0 Guidance Document Page 2

FSMA Snap Shot Signed into law January 4, 2011 – The current food safety system has opportunity for improvement. • 1 in 6 Americans (48 million) sickened; 128, 000 hospitalized; 3, 000 die each year from foodborne diseases (CDC, 2011) – Identified by FDA as the most sweeping reform of food safety laws in more than 70 years. • GOAL: Aims to ensure the U. S. food supply is safe by shifting the focus of federal regulators from responding to contamination to preventing it. Page 3

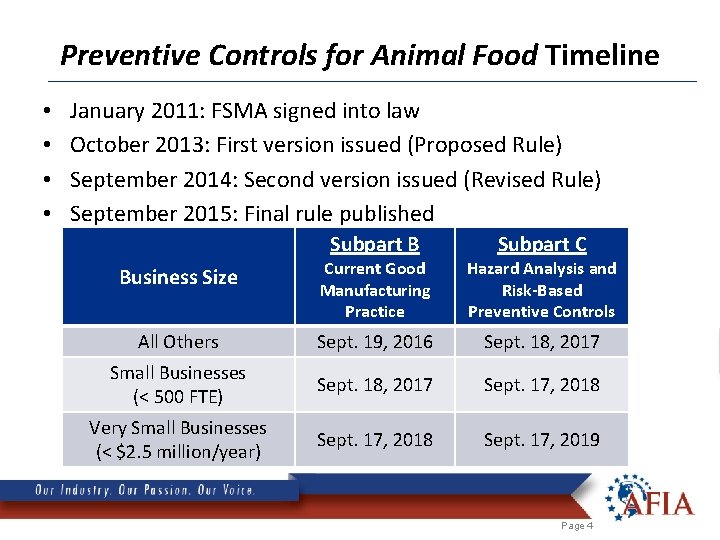
Preventive Controls for Animal Food Timeline • • January 2011: FSMA signed into law October 2013: First version issued (Proposed Rule) September 2014: Second version issued (Revised Rule) September 2015: Final rule published Subpart B Subpart C Business Size Current Good Manufacturing Practice Hazard Analysis and Risk-Based Preventive Controls All Others Sept. 19, 2016 Sept. 18, 2017 Small Businesses (< 500 FTE) Sept. 18, 2017 Sept. 17, 2018 Very Small Businesses (< $2. 5 million/year) Sept. 17, 2018 Sept. 17, 2019 Page 4

Who Must Comply? • Facilities that manufacture, process, pack, or hold animal food for consumption in the United States – In general, those that register under Section 415 of the Federal Food, Drug, and Cosmetic Act (Bioterrorism Act). – Not complying is considered a prohibited act. • Animal food covered by specific CGMP regulations must still comply with those regulations – Low-acid canned food – Medicated feed Page 5

21 CFR Part 507 Preventive Controls for Animal Food • Subpart A – General Provisions • Subpart B – Current Good Manufacturing Practice • Subpart C – Hazard Analysis and Risk-Based Preventive Controls • Subpart D – Withdrawal of a Qualified Facility Exemption • Subpart E – Supply-Chain Program • Subpart F – Requirements Applying to Records That Must Be Established and Maintained Page 6

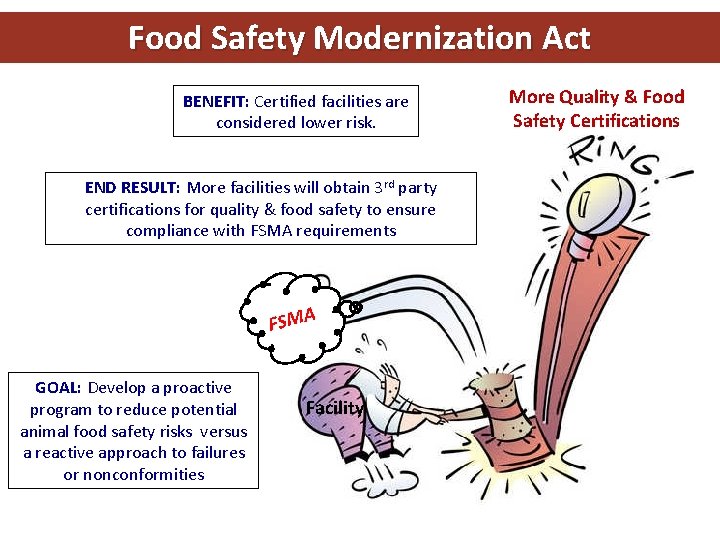
Food Safety Modernization Act BENEFIT: Certified facilities are considered lower risk. More Quality & Food Safety Certifications END RESULT: More facilities will obtain 3 rd party certifications for quality & food safety to ensure compliance with FSMA requirements FSMA GOAL: Develop a proactive program to reduce potential animal food safety risks versus a reactive approach to failures or nonconformities Facility Page 7

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 Good News! Requirements for FSC 36 Safe Feed/Safe Food are not changing. However, the expectations relative to FSMA compliance are clearer. Page 8

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 More direct references to FSMA animal food rule requirements • After comparing FSMA animal food rule to FSC 36 guidance document, there seemed to be several gaps. • CGMP requirements were not specifically identified although the expectations were generally called out within the various requirements. • The requirements for records (subpart F of the animal food rule) have been incorporated into clause 2. 3 Records. • FSMA requirements for the food safety plan and preventive controls have been added to the guidance document. Page 9

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 “Auditing Guidance” was removed while “Implementation Guidance” was maintained • Within version 7. 0, the auditing guidance will be removed because this has been a point of confusion for auditors as well as facilities seeking certification. • Many auditors and auditees have interpreted this information as the only items that are relevant to the audit • Auditing guidance will be an individual/stand-alone document. This allows changes or additions as needed to help auditors maintain consistency and improve the quality of audits. Page 10

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 Reference to clauses has been updated • In version 6. 0, there were sections with the same number within the document. As an example, Section 2. 3 = “Auditing Duration Guide” while within the scheme requirements in Section 5. 0, 2. 3 = “Document Control (M)”. • In version 7. 0, “SF/SF” will be added in front of each clause for Safe Feed/Safe Food requirements in Section 5. 0. Page 11

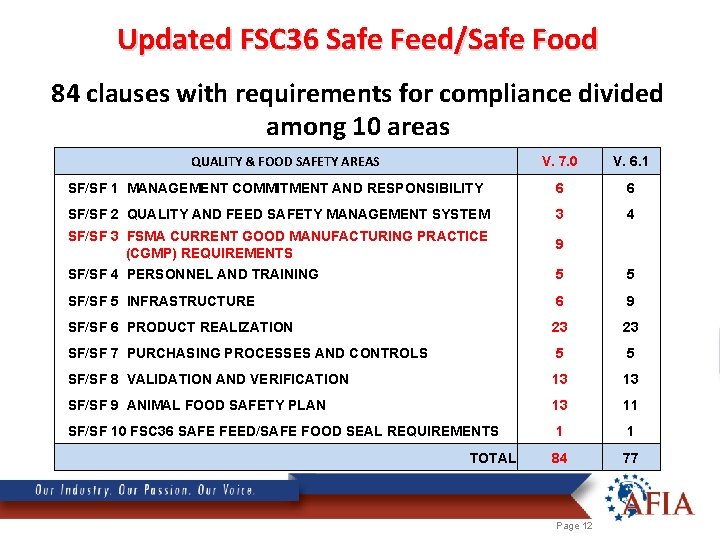
Updated FSC 36 Safe Feed/Safe Food 84 clauses with requirements for compliance divided among 10 areas QUALITY & FOOD SAFETY AREAS V. 7. 0 V. 6. 1 SF/SF 1 MANAGEMENT COMMITMENT AND RESPONSIBILITY 6 6 SF/SF 2 QUALITY AND FEED SAFETY MANAGEMENT SYSTEM 3 4 SF/SF 3 FSMA CURRENT GOOD MANUFACTURING PRACTICE (CGMP) REQUIREMENTS 9 SF/SF 4 PERSONNEL AND TRAINING 5 5 SF/SF 5 INFRASTRUCTURE 6 9 SF/SF 6 PRODUCT REALIZATION 23 23 SF/SF 7 PURCHASING PROCESSES AND CONTROLS 5 5 SF/SF 8 VALIDATION AND VERIFICATION 13 13 SF/SF 9 ANIMAL FOOD SAFETY PLAN 13 11 SF/SF 10 FSC 36 SAFE FEED/SAFE FOOD SEAL REQUIREMENTS 1 1 84 77 TOTAL Page 12

Page 13

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 1 MANAGEMENT COMMITMENT AND RESPONSIBILITY SF/SF 1. 1 Management Policy (M) SF/SF 1. 2 Management Responsibility (M) Remains the same with 2017 update SF/SF 1. 3 Responsibility, Authority and Communication (M) SF/SF 1. 4 Management Review SF/SF 1. 4. 1 Management Review Process SF/SF 1. 4. 2 Management Review Inputs and Outputs SF/SF 1. 4. 3 Records for Management Review OBJECTIVE: Ensure management commitment and engagement with the quality and animal food safety program Page 14

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 2 QUALITY AND FEED SAFETY MANAGEMENT SYSTEM SF/SF 2. 1 General Requirements SF/SF 2. 2 Quality and Feed Safety Manual (M) Clause removed in 2017 update SF/SF 2. 3 Document Control (M) SF/SF 2. 4 Records (M) Updated with FSMA language (more detail) OBJECTIVE: Ensure the facility maintains the “tools” to implement an effective quality and animal food safety program Page 15

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 New Clau s es A d ded to C over CGM P’s Page 16

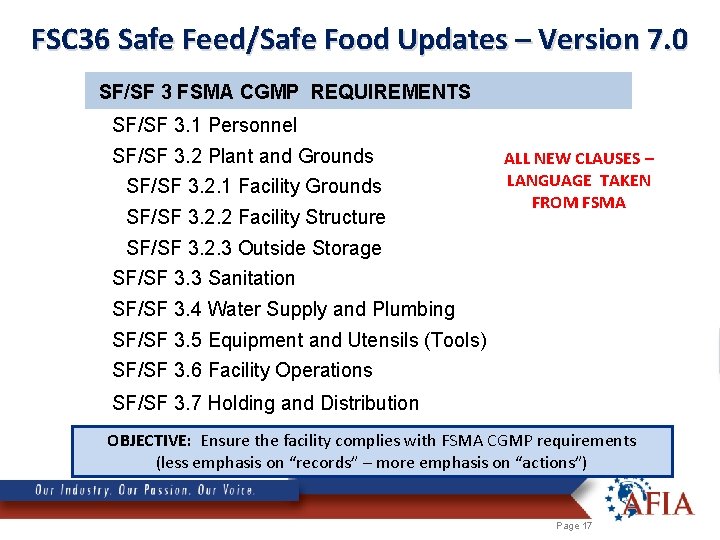
FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 3 FSMA CGMP REQUIREMENTS SF/SF 3. 1 Personnel SF/SF 3. 2 Plant and Grounds SF/SF 3. 2. 1 Facility Grounds SF/SF 3. 2. 2 Facility Structure ALL NEW CLAUSES – LANGUAGE TAKEN FROM FSMA SF/SF 3. 2. 3 Outside Storage SF/SF 3. 3 Sanitation SF/SF 3. 4 Water Supply and Plumbing SF/SF 3. 5 Equipment and Utensils (Tools) SF/SF 3. 6 Facility Operations SF/SF 3. 7 Holding and Distribution OBJECTIVE: Ensure the facility complies with FSMA CGMP requirements (less emphasis on “records” – more emphasis on “actions”) Page 17

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 4 PERSONNEL AND TRAINING SF/SF 4. 1 Competency and Job Descriptions (M) SF/SF 4. 2 Requirements for a Preventive Controls Qualified Individual (PCQI) (M) NEW SF/SF 4. 3 Personnel Policies and Behavior SF/SF 4. 4 Personnel Facilities SF/SF 4. 5 Visitors OBJECTIVE: Ensure the personnel are properly prepared, facilities are adequate and visitors are controlled Page 18

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 5 INFRASTRUCTURE SF/SF 5. 1 Maintenance REMOVED SF/SF 4. 1 Facility Construction and Surfaces SF/SF 4. 2. 1 Equipment SF/SF 4. 6 Exterior SF/SF 5. 2 Lighting and Work Areas SF/SF 5. 3 Pest Management and Control SF/SF 5. 3. 1 Pest Management (M) SF/SF 5. 3. 2 Pest Control Chemicals (M) SF/SF 5. 3. 3 Pest Management Personnel SF/SF 5. 4 Cleaning and Housekeeping (M) OBJECTIVE: Ensure the facility is properly designed and maintained for its intended purpose, ensure work areas are sufficient and pest management is properly implemented Page 19

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 6 PRODUCT REALIZATION No changes in Product Realization SF/SF 6. 1 Product Development SF/SF 6. 2 Packaging and Raw Materials Receiving Processes SF/SF 6. 2. 1 Receiving Processes for Packaging Materials SF/SF 6. 2. 2 Receiving Processes for Raw Materials and Ingredients SF/SF 6. 2. 3 Equipment at Receiving SF/SF 6. 3 Manufacturing Processes SF/SF 6. 3. 1 Process Control (M) SF/SF 6. 3. 2 Control of Raw Materials and Ingredients SF/SF 6. 3. 3 Product Release (M) SF/SF 6. 4 Finished Products SF/SF 6. 4. 1 Finished Products Specifications (M) SF/SF 6. 4. 2 Product Formulation (M) Page 20

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 6 PRODUCT REALIZATION No changes in Product Realization SF/SF 6. 5 Customer Related Processes SF/SF 6. 5. 1 Customer Requirements SF/SF 6. 5. 2 Customer Communication SF/SF 6. 6 Labeling (M) SF/SF 6. 7 Nonconforming Products and Materials (M) SF/SF 6. 8 Rework (M) SF/SF 6. 9 Inventory Stock Rotation Page 21

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 6 PRODUCT REALIZATION No changes in Product Realization SF/SF 6. 10 Storage of Materials and Finished Products SF/SF 6. 10. 1 Storage of Raw Materials and Ingredients SF/SF 6. 10. 2 Storage of Packaging SF/SF 6. 10. 3 Storage of Finished Products SF/SF 6. 10. 4 Storage of Nonconforming Materials SF/SF 6. 10. 5 Bulk Storage of Ingredients and Finished Products SF/SF 6. 11 Storage of Hazardous Chemicals SF/SF 6. 11. 1 Hazardous Chemical Storage Process (M) OBJECTIVE: Ensure food safety concerns are considered and records maintained SF/SF 6. 11. 2 Hazardous Chemical Area (M)finished for developing products, receiving materials. Storage and manufacturing products; ensure appropriate information is received from and provided to SF/SF 6. 12 Loading, Transport and Unloading Practices customers; ensure food safety is maintained during storage Page 22

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 7 PURCHASING PROCESSES AND CONTROLS SF/SF 7. 1 Vendors for Incoming Goods and Services SF/SF 7. 1. 1 Approved Vendors (M) No changes in Purchasing Processes and Controls SF/SF 7. 1. 2 Unapproved Vendors or Temporary Sourcing SF/SF 7. 2 Raw and Packaging Materials Specifications (M) SF/SF 7. 3 Contract Service Providers SF/SF 7. 3. 1 Specifications for Contract Service Providers SF/SF 7. 3. 2 Contract Manufacturing OBJECTIVE: Ensure processes are implemented for vendor (supplier) approval and that appropriate communications are maintained to provide the desired materials Page 23

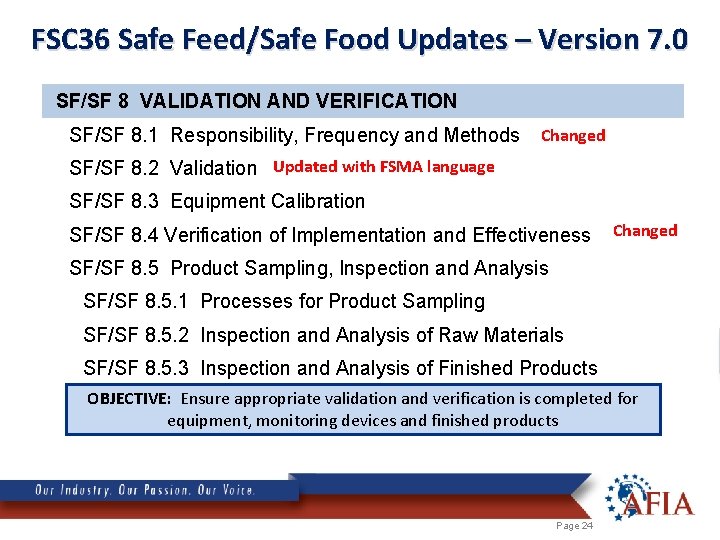
FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 8 VALIDATION AND VERIFICATION SF/SF 8. 1 Responsibility, Frequency and Methods Changed SF/SF 8. 2 Validation Updated with FSMA language SF/SF 8. 3 Equipment Calibration SF/SF 8. 4 Verification of Implementation and Effectiveness Changed SF/SF 8. 5 Product Sampling, Inspection and Analysis SF/SF 8. 5. 1 Processes for Product Sampling SF/SF 8. 5. 2 Inspection and Analysis of Raw Materials SF/SF 8. 5. 3 Inspection and Analysis of Finished Products OBJECTIVE: Ensure appropriate validation and verification is completed for equipment, monitoring devices and finished products Page 24

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 8 VALIDATION AND VERIFICATION SF/SF 8. 6 Internal Audits No changes SF/SF 8. 6. 1 Internal Audit Process SF/SF 8. 6. 2 Internal Auditors SF/SF 8. 6. 3 Internal Audit Corrective Actions SF/SF 8. 7 Product Identification (M) SF/SF 8. 8 Product Traceability (M) SF/SF 8. 9 Animal Food Defense and Biosecurity Plan OBJECTIVE: Ensure appropriate validation and verification is completed for equipment, monitoring devices and finished products through internal auditing, product identification and traceability; ensure an animal defense/biosecurity plan has been developed Page 25

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 9 ANIMAL FOOD SAFETY PLAN Updated with FSMA language SF/SF 9. 1 Components of a Food Safety Plan (M) SF/SF 9. 2 Records for the Food Safety Plan (M) SF/SF 9. 3 Hazard Analysis (M) SF/SF 9. 4 Preventive Controls (M) SF/SF 9. 5 Corrective and Preventive Actions (M) SF/SF 9. 6 Supply-Chain Program for FSMA Subpart E Compliance SF/SF 9. 7 When Implementation of Preventive Controls Is Not Required OBJECTIVE: Ensure an animal food safety plan has been developed CAUTION: Animal Food Safety Plan is not required by FSMA until Sept 2017 for Large Businesses and Sept 2018 for Small Businesses Page 26

FSC 36 Safe Feed/Safe Food Updates – Version 7. 0 SF/SF 10 FSC 36 SAFE FEED/SAFE FOOD SEAL REQUIREMENTS SF/SF 10. 1 Compliance with Safe Feed/Safe Food Seal Licensing Agreement OBJECTIVE: Ensure the Safe Feed/Safe Food logo is used appropriately and proper statements are provided on packaging or labels Page 27

Page 28

THANK YOU