Petrology definition and Scope 1 What is Petrology




















- Slides: 31

Petrology : definition and Scope 1. What is Petrology? -Study of Rocks 2. What Study? -Source and Processes 3. How to Study? -Field Relations, Field characteristics, Mineralogical (what are the minerals? ), Petrographical ( textural: mineral size, mineral shape, mineral mutual relationships) and Chemical composition (Major elements, Trace elements, Isotopic compositions), time of formation of rock (geochronology)


• What is a rock? • A rock is simply a naturally occurring consolidated mixture of minerals. • Rocks - A rock is a solid aggregate of one or more minerals that have been cohesively brought together by a rockforming process. • What do we mean when we say that rock is consolidated? • In general, rocks are harder, and in many cases, much harder, than the soils that blanket the Earth’s surface.

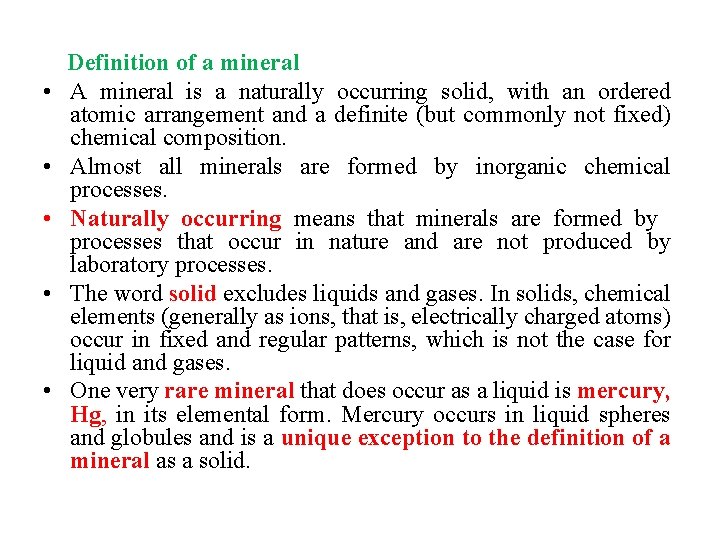
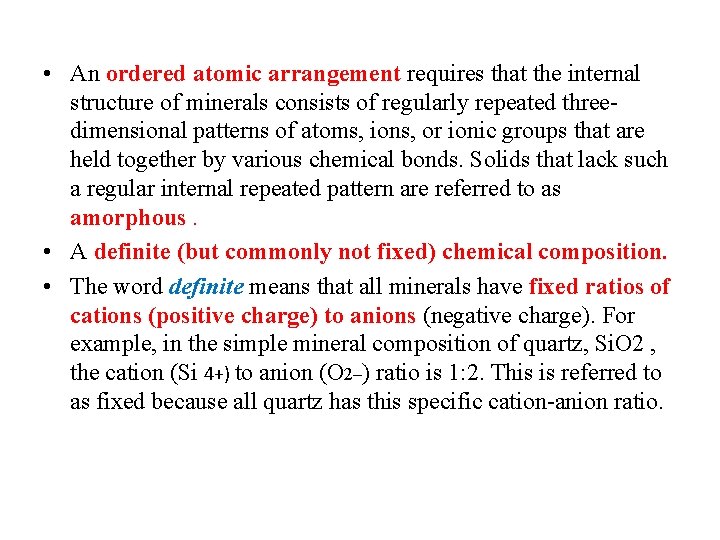
• • • Definition of a mineral A mineral is a naturally occurring solid, with an ordered atomic arrangement and a definite (but commonly not fixed) chemical composition. Almost all minerals are formed by inorganic chemical processes. Naturally occurring means that minerals are formed by processes that occur in nature and are not produced by laboratory processes. The word solid excludes liquids and gases. In solids, chemical elements (generally as ions, that is, electrically charged atoms) occur in fixed and regular patterns, which is not the case for liquid and gases. One very rare mineral that does occur as a liquid is mercury, Hg, in its elemental form. Mercury occurs in liquid spheres and globules and is a unique exception to the definition of a mineral as a solid.

• An ordered atomic arrangement requires that the internal structure of minerals consists of regularly repeated threedimensional patterns of atoms, ions, or ionic groups that are held together by various chemical bonds. Solids that lack such a regular internal repeated pattern are referred to as amorphous. • A definite (but commonly not fixed) chemical composition. • The word definite means that all minerals have fixed ratios of cations (positive charge) to anions (negative charge). For example, in the simple mineral composition of quartz, Si. O 2 , the cation (Si 4+) to anion (O 2–) ratio is 1: 2. This is referred to as fixed because all quartz has this specific cation-anion ratio.

Examples of some familiar minerals • Olivine, pyroxene, amphibole, Biotite, Muscovite, Microcline, orthoclase, plagioclase, calcite, halite, quartz, talc, chrysotile (one of several “asbestos” minerals), Zircon, and garnet. Common Rock Forming Min 1. Silicates 2. Non Silicates • Approximately 4150 minerals are known, of which 1140 are silicates; 624, sulfides and sulfosalts; 458, phosphates; 411, oxides and hydroxides; 234, carbonates; and 90, native elements.

1. SILICATE MINERALS • The widespread occurrence of silicate minerals makes them by far the most important group of rock forming minerals; • The unifying characteristic of silicate minerals is the presence of silica tetrahedra (Si. O 4)− 4 composed of silica (Si+4 ) in tetrahedral coordination with oxygen (O− 2 ). • Silicates – made of silicon and oxygen and make up 92 % of Earth’s crust. • The basic structural unit of all silicates is silica tetrahedron, formed by a silicon cation (Si 4+) closely surrounded by four oxygen anions (O 2 - ) • Silicates divided into two: 1. Ferromagnesian Silicates 2. Nonferromagnesiam Silicates

1. Ferromagnesian Silicates • made of iron, magnesium, and silicates • Form a basic tetrahederal structure. • Higher density and darker color than other silicates due to the presence of iron and magnesium • Ex of ferromagnesian silicates: olivine, augite, hornblende , and biotite 2. Nonferromagnesian Silicates • silicates that do not contain either iron or magnesium. • Lower density and lighter color than the ferromagnesian silicates. • Ex of nonferromagnesian silicates: Muscovite, orthoclase and quartz

2. NON- SILICATE MINERALS • With the exception of native elements, non -silicate minerals are classified by their major anionic group. • Although non – silicate minerals are far less abundant in Earth ’ s crust and mantle than are silicate minerals, many are of great economic value. This makes knowledge of non - silicate minerals extremely important to practicing Earth scientists. • Native elements • Sulfides • Sulfosalts • Oxides • Hydroxides • Halides • Carbonates • Phosphates • Sulfates

• Native elements divided into three groups 1. metals (metals : subdivided into three groups: the gold group, the platinum group and the iron group) 2. semi - metals, 3. non - metals. • Minerals composed of a single element are called native elements. This group also includes several minerals that are composed of two or more closely related elements that possess very similar chemical characteristics. • Fewer than 20 elements occur in their native state. Most native element minerals are quite rare; many have only been discovered with the advent of sophisticated instrumentation for examining Earth materials.

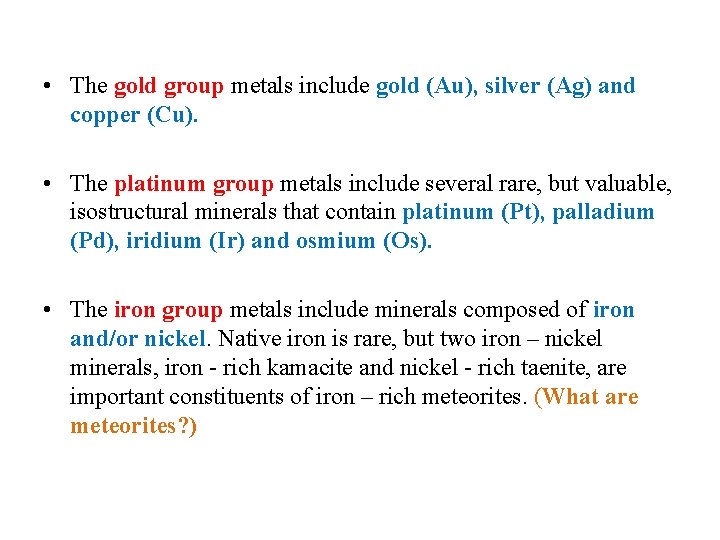
1. Metals • The native metals are composed of metallic elements. • All native metals crystallize in the isometric system, and almost all are characterized by cubic closest packing with face centered cubic crystal lattices. • As a result, substitution solid solution of metals of similar radii (e. g. , gold and silver, iron and nickel) is common. • Native metals are subdivided into three groups: the gold group, the platinum group and the iron group. • These groups are distinguished on the basis of the chemical nature of the metallic elements and the properties they confer on their members.

• The gold group metals include gold (Au), silver (Ag) and copper (Cu). • The platinum group metals include several rare, but valuable, isostructural minerals that contain platinum (Pt), palladium (Pd), iridium (Ir) and osmium (Os). • The iron group metals include minerals composed of iron and/or nickel. Native iron is rare, but two iron – nickel minerals, iron - rich kamacite and nickel - rich taenite, are important constituents of iron – rich meteorites. (What are meteorites? )

2. Semi - metals • The native semi - metals are composed of semi -metallic elements such as arsenic (As), antimony (Sb) and bismuth (Bi). 3. Non - metals • The native non - metals are composed of non -metallic elements, chiefly sulfur and carbon. • Diamonds are the hardest minerals on Earth due to short, strong covalent bonds that bind atoms tightly in its cubic closest packing structure. • Graphite, on the other hand, is very soft because some of its atoms are bound together by van der Waals forces.

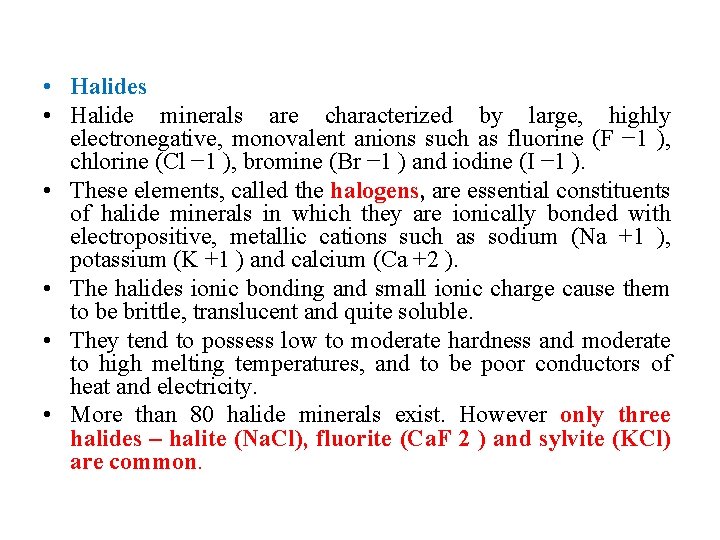
• Halides • Halide minerals are characterized by large, highly electronegative, monovalent anions such as fluorine (F − 1 ), chlorine (Cl − 1 ), bromine (Br − 1 ) and iodine (I − 1 ). • These elements, called the halogens, are essential constituents of halide minerals in which they are ionically bonded with electropositive, metallic cations such as sodium (Na +1 ), potassium (K +1 ) and calcium (Ca +2 ). • The halides ionic bonding and small ionic charge cause them to be brittle, translucent and quite soluble. • They tend to possess low to moderate hardness and moderate to high melting temperatures, and to be poor conductors of heat and electricity. • More than 80 halide minerals exist. However only three halides – halite (Na. Cl), fluorite (Ca. F 2 ) and sylvite (KCl) are common.

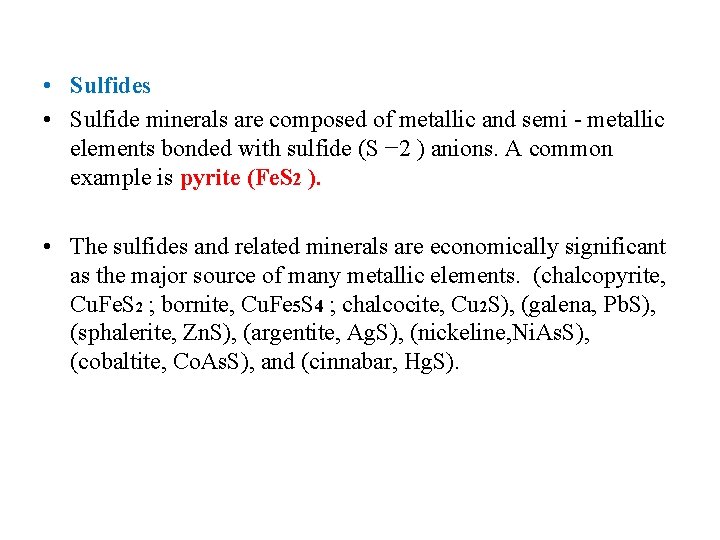
• Sulfides • Sulfide minerals are composed of metallic and semi - metallic elements bonded with sulfide (S − 2 ) anions. A common example is pyrite (Fe. S 2 ). • The sulfides and related minerals are economically significant as the major source of many metallic elements. (chalcopyrite, Cu. Fe. S 2 ; bornite, Cu. Fe 5 S 4 ; chalcocite, Cu 2 S), (galena, Pb. S), (sphalerite, Zn. S), (argentite, Ag. S), (nickeline, Ni. As. S), (cobaltite, Co. As. S), and (cinnabar, Hg. S).

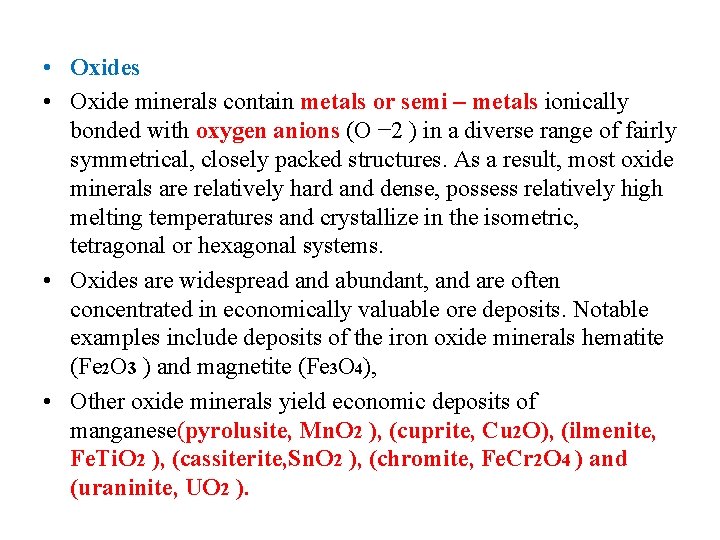
• Oxides • Oxide minerals contain metals or semi – metals ionically bonded with oxygen anions (O − 2 ) in a diverse range of fairly symmetrical, closely packed structures. As a result, most oxide minerals are relatively hard and dense, possess relatively high melting temperatures and crystallize in the isometric, tetragonal or hexagonal systems. • Oxides are widespread and abundant, and are often concentrated in economically valuable ore deposits. Notable examples include deposits of the iron oxide minerals hematite (Fe 2 O 3 ) and magnetite (Fe 3 O 4), • Other oxide minerals yield economic deposits of manganese(pyrolusite, Mn. O 2 ), (cuprite, Cu 2 O), (ilmenite, Fe. Ti. O 2 ), (cassiterite, Sn. O 2 ), (chromite, Fe. Cr 2 O 4 ) and (uraninite, UO 2 ).

• Hydroxides • The defining components of hydroxide minerals are metallic elements in combination with hydroxyl (OH− 1 ) ion. Oxygen is also an essential component of many hydroxide minerals. Hydroxide minerals tend to be softer than oxides and possess somewhat lower specific gravity. • Hydroxide minerals commonly develop by the weathering and alteration of other minerals under near surface conditions. • The most economically important hydroxide minerals are those that belong to the bauxite mineral group, which is the major source of aluminum ore. They include boehmite (Al. O(OH)), diaspore (Al. O(OH)) and gibbsite [Al(OH)3 ]. • Another significant hydroxide mineral is the iron - bearing goethite [Fe. O(OH)], which is an important iron ore mineral in lateritic soils and in bog iron deposits. • Other important hydroxide minerals include the manganese minerals brucite [Mn(OH)2 ], manganite [Mn. O 2(OH)2 ] and romanechite [Ba. Mn 9 O 20 • 3 H 2 O].

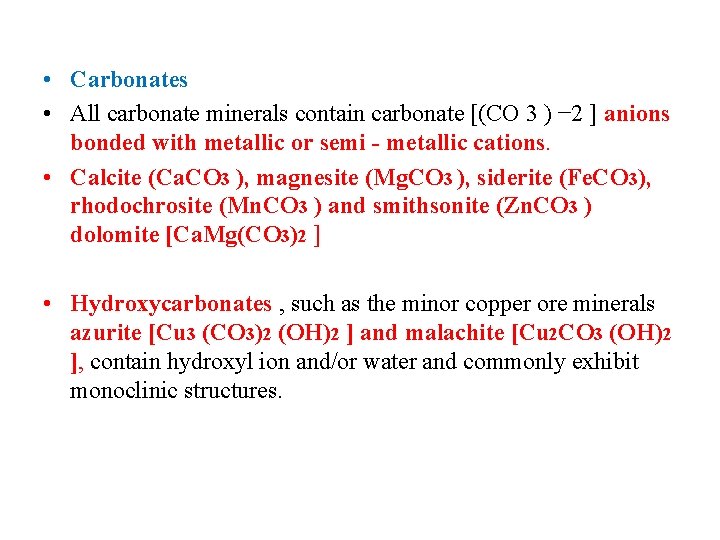
• Carbonates • All carbonate minerals contain carbonate [(CO 3 ) − 2 ] anions bonded with metallic or semi - metallic cations. • Calcite (Ca. CO 3 ), magnesite (Mg. CO 3 ), siderite (Fe. CO 3), rhodochrosite (Mn. CO 3 ) and smithsonite (Zn. CO 3 ) dolomite [Ca. Mg(CO 3)2 ] • Hydroxycarbonates , such as the minor copper ore minerals azurite [Cu 3 (CO 3)2 (OH)2 ] and malachite [Cu 2 CO 3 (OH)2 ], contain hydroxyl ion and/or water and commonly exhibit monoclinic structures.

• Sulfates are minerals that contain (SO 4 )− 2 combined with one or more metals or semi -metals. • Major groups of sulfate minerals include hydrated sulfates , of which the most common example is gypsum (Ca. SO 4 • 2 H 2 O), and anhydrous sulfates , of which the most common example is anhydrite (Ca. SO 4 ). • Gypsum varieties include selenite , which is composed of large macroscopic crystals; satinspar, which consists of fibrous aggregates of parallel acicular – capillary crystals; and alabaster, which consists of masses of randomly oriented microsopic crystals. Barite (Ba. SO 4 )

• Phosphates • Phosphate minerals contain (PO 4 )− 3 anions bonded with metal cations. • Although the phosphate group is large, only two minerals – apatite and monazite – are relatively common. • Apatite [Ca 5 (PO 4)3 (Cl, F, OH)], Monazite [(Ce, Y, La, Th)PO 4 ], the other important phosphate mineral, is the principal source of Th.

• Common Minerals of Ig rocks : Olivine, pyroxene, amphibole, biotite, muscovite, Feldspar (= microcline, orthoclase, plagioclase), quartz • Common Minerals of Sedimentary rocks : Quartz, feldspar, Muscovite, calcite, dolomite • Common Minerals of Metamorphic rocks: Garnet, Andalusite, Kyanite, sillimanite, cordierite, staurolite, talc, tremolite

• • • Chemical composition of Common Minerals Quartz : Si. O 2 Feldspar Group: 1. Plagioclase 2. K-Feldspar 1. Plagioclase: solid solution b/w Albite (Na. Al. Si 3 O 8) and anortite (Ca. Al 2 Si 2 O 8) 2. K-feldspar : Orthoclase (KAl. Si 3 O 8), Microcline (KAl. Si 3 O 8) Mica Group (K and water bearing) : Muscovite (K Al 2 (Al. Si 3)O 10) (OH)2, Biotite (K (Mg, Fe)3 (Al Si 3) O 10) (OH)2 Olivine : (Mg, Fe)2 Si. O 4 Pyroxene Group : Silicate of Ca, Mg, Fe Hornblende = complex silicate of Mg, Fe, Ca, Na, Al

ORE FORMING MINERALS • Ore minerals : Ore minerals are minerals from which metals or other elements can be profitably recovered. • Cu ore: Malachite: Cu 2 CO 3(OH)2, Azurite Cu 3(CO 3)2(OH)2, Chalcocite Cu. Fe. S 2, Bornite Cu 5 Fe. S 4 • Manganese ore: pyrolusite (Mn. O 2 ), psilomelane (hydrous manganese oxide with variable amounts of barium and potassium) • Tin Ore: Cassiterite Sn. O 2 • Pb ore: Galena Pb. S • Zn Ore: Sphalerite Zn. S • Al ore: Bauxite

• Fe Ore: BIF (rock), Laterite (Rock), Limonite (finely granular, sometimes amorphous, mixtures of iron hydroxide and hydrated iron hydroxide minerals). Such granular masses are typically soft, with a characteristic rusty yellow to yellow - brown color, Hematite Fe 2 O 3, Magnetite Fe 3 O 4, Siderite Fe. CO 3 • Sulphur ore: Pyrite Fe. S 2 • Cr Ore: Chromite Fe. Cr 2 O 4 • U/Th ore = Uraninite, pitchblende, Carnotite, Thorianite, thorite, monazite • • • Molybdenum ore : Molybdenite: Mo. S 2 Ni ore: Pentlandite: (Fe, Ni)9 S 8 Silver ore : argentite (Ag. S), Nickel ore nickeline (Ni. As. S), Cobalt ore : cobaltite (Co. As. S), Mercury ore : cinnabar (Hg. S).

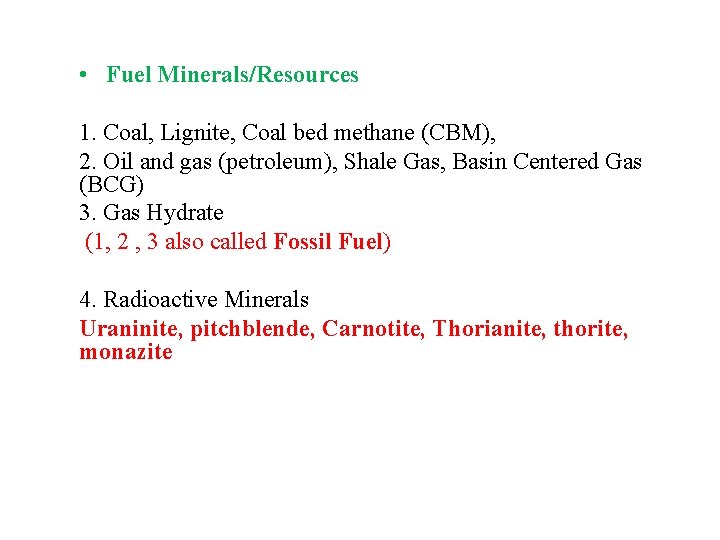
• Fuel Minerals/Resources 1. Coal, Lignite, Coal bed methane (CBM), 2. Oil and gas (petroleum), Shale Gas, Basin Centered Gas (BCG) 3. Gas Hydrate (1, 2 , 3 also called Fossil Fuel) 4. Radioactive Minerals Uraninite, pitchblende, Carnotite, Thorianite, thorite, monazite

• Industrial Min/rocks : Min/rocks that are used for different industries • Min for chemical industry : sulfur, pyrite, barite • Ceramic: gypsum, talc, feldspar, glass sand (pure quartz) • Fertilizer: Phosphorite, rock phosphase • Refractory : Graphite, dolomite, magnesite, kyanite, sillimanite, andalusite, fire clay • Construction Industry: limestone, granite, marble, dolerite, sandstone, sand, gravel • Insulation and electrical : Mica, asbestos • Gemstones : Diamond, corundum, beryl, Topaz, zircon, garnet • Cement : limestone, gypsum • Iron : BIF, laterite • Aluminium : Baxuite • Glass : glass sand (quatz), feldspar • Drilling : diamond

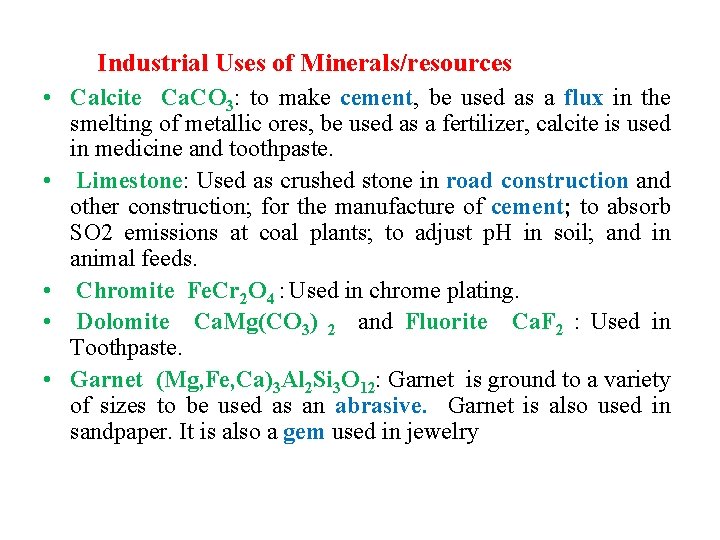
Industrial Uses of Minerals/resources • Calcite Ca. CO 3: to make cement, be used as a flux in the smelting of metallic ores, be used as a fertilizer, calcite is used in medicine and toothpaste. • Limestone: Used as crushed stone in road construction and other construction; for the manufacture of cement; to absorb SO 2 emissions at coal plants; to adjust p. H in soil; and in animal feeds. • Chromite Fe. Cr 2 O 4 : Used in chrome plating. • Dolomite Ca. Mg(CO 3) 2 and Fluorite Ca. F 2 : Used in Toothpaste. • Garnet (Mg, Fe, Ca)3 Al 2 Si 3 O 12: Garnet is ground to a variety of sizes to be used as an abrasive. Garnet is also used in sandpaper. It is also a gem used in jewelry

• Gypsum Ca. SO 4 • 2 H 2 O: Gypsum is used in wallboard and plaster products. It is also used to make Portland cement and has some agricultural applications. A small amount of very pure gypsum is used in glass making. Used in plaster of paris. Gypsum is used as fake snow in movies. • Halite Na. Cl: Halite is used for table salt • Hematite Fe 2 O 3 Hematite is a common ore of iron because it contains about 70% iron. To make steel. Powdered hematite is often used as a pigment. • Kaolinite Al 2 Si 2 O 5(OH)4 Kaolinite is mostly used in the paper industry. It is a filler making the paper better for printing and cheaper. And it coats the paper also making it better for printing, enhancing the colors. In the ceramics industry, kaolinite is used to make the clay very white. Kaolinite, like the feldspars, is also used as a filler in things like paints, rubber, plastics, adhesives, etc. Since it is extremely fine, it can easily be mixed with water and transported. It is used in medicine, bricks, toothpaste, cosmetics, and as a food additive. It is also used in a spray applied to fruits and vegetables to deter insect damage.

• Muscovite KAl 2 Al. Si 3 O 10(OH)2 used as an insulator in electrical appliances. Ground mica is often used in paint because of its light weight and flat shape. • Micas have their main industrial use in the field of electronics. It is used as an insulating material since is does not transfer heat or energy very well. Mica is being used more and more in areas of very high heat levels such as rockets and missiles, in production of fireproofing and insulating materials. in well-drilling muds, and in plastics, roofing, rubber, and welding rods. • Orthoclase KAl. Si 3 O 8 and Plagioclase (Na, Ca)(Al, Si)4 O 8 Used in glassmaking. It helps lower the melting point of quartz, which is used to make glass. Feldspar is a leading ingredient in the manufacture of glass and ceramics. Specifically, feldspar and other minerals are used in glazing pottery where they are applied as a powder or slurry and then melted onto the pot as a glass when fired in a kiln. • Potassium Feldspar (Orthoclase): When it is combined with kaolin and quartz it is used to manufacture porcelain, sometimes used for high-tension electrical insulators and dental products.

• Quartz Si. O 2 One use of quartz is to control frequency in some radios. It's also used in timers in electronic equipments, such as computers. § Quartz can be used in various electronic appliances such as cell phones and computers, along with pressure gauges and oscillators because it is piezoelectric, which means that it is polarized under pressure. This same thing happens by changing the temperature, which is called pyroelectric. § Quartz: Used for electronic timing (it vibrates at a very specific frequency when a current is passed through it) § The various types of Quartz can be use simply as a gemstone in jewelry, one of which is amethyst § Quartz is used in clocks and watches, in microprocessor chips in personal computers, and in telecommunications equipment.

• Sylvite KCl: Used as an ingredient in fertilizer, especially for citrus fruits, which are chloride sensitive; as a drill mud additive; as a water softener; and a substitute for salt. • Talc Mg 3 Si 4 O 10(OH) Talc has several applications. One is for use in sewage treatment plants, where it binds to bacteria, causing them to be precipitated from the water. The byproduct of this is fertilizer. Talc is also used to make talcum powder, among other things. Talc is used as an ingredient in ceramics, paper, paint, roofing, plastics, cosmetics, talcum, and baby powder. • Asbestos : insulation and heat and fire resistant industry • Graphite: lead pencil, batteries
 Petrology is the study of
Petrology is the study of Product scope vs project scope
Product scope vs project scope Use case diagram
Use case diagram Scope and limitation meaning
Scope and limitation meaning Scope of educational psychology slideshare
Scope of educational psychology slideshare Pharmacognacy
Pharmacognacy Medical social work definition
Medical social work definition Definition of clinical pharmacy
Definition of clinical pharmacy Objek ilmu pendidikan
Objek ilmu pendidikan Psycholinguistics scope
Psycholinguistics scope Define research scope
Define research scope Scope of business meaning
Scope of business meaning Eal continuum
Eal continuum Orton gillingham manipulatives
Orton gillingham manipulatives Purpose and scope example
Purpose and scope example Nature and scope of physics
Nature and scope of physics Importance of bioinformatics
Importance of bioinformatics Project charter and scope statement
Project charter and scope statement Purpose and scope example
Purpose and scope example Scope and development of pharmacognosy
Scope and development of pharmacognosy The modern approach to financial management view
The modern approach to financial management view Megawords scope and sequence
Megawords scope and sequence What is marketing nature scope and importance
What is marketing nature scope and importance Discuss the nature and scope of managerial economics
Discuss the nature and scope of managerial economics Management accounting
Management accounting Research limitation example
Research limitation example Significance of the study example
Significance of the study example Ethical capability
Ethical capability Accounting scope and objectives
Accounting scope and objectives What is channel management decisions
What is channel management decisions Research proses
Research proses The scope and method of economics chapter 1
The scope and method of economics chapter 1