Pharmacoepidemiology Definition and scope Definition and scope Pharmacoepidemiology

















- Slides: 23

Pharmacoepidemiology Definition and scope

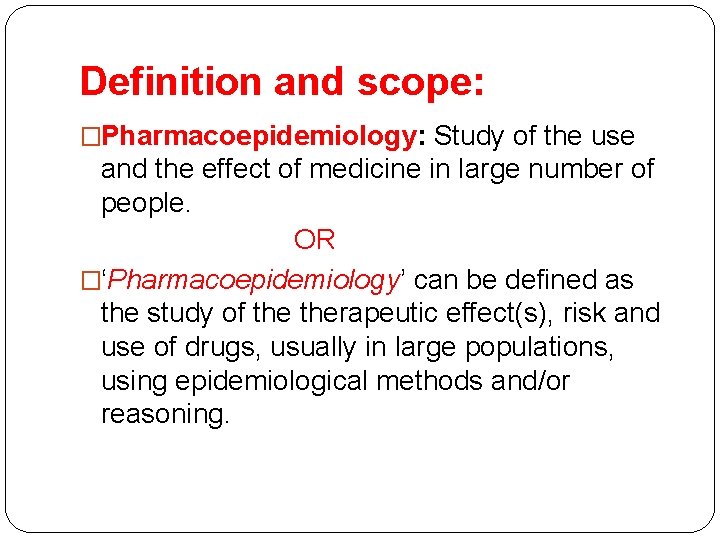
Definition and scope: �Pharmacoepidemiology: Study of the use and the effect of medicine in large number of people. OR �‘Pharmacoepidemiology’ can be defined as the study of therapeutic effect(s), risk and use of drugs, usually in large populations, using epidemiological methods and/or reasoning.

Epidemiology: �Epidemiology Study of the relationships between diseases or any other biological phenomenon and various factors (e. g. lifestyle, environment or social setting, individual traits, etc. ) which can influence their frequency, distribution and evolution. �Σ Descriptive epidemiology, in which the objective is to describe a population (e. g. drug utilization studies), �Σ Analytic epidemiology, in which the objective is to study the associations (causal or otherwise) that can exist, within a population, between the occurrence of an event and a given characteristic

�Pharmacoepidemiology: Bridging b/w clinical pharmacology and epidemiology �Application of the principles of epidemiology to drug effect and drug use �Better assessment of risk/benefit balance for the use of any particular drug in any particular patient Focus: �Clinical Pharmacology: Drug effect in Individual Patient �Drug Utilization: Drug usage pattern and appropriateness of drug use in groups �Pharmacoepidemiology: Relation between drug exposure and health outcomes in defined

Adverse reactions � Type A reactions: reactions tend to be common, dose- related, predictable, and less serious. They can usually be treated by simply reducing the dose of the drug. � They tend to occur in individuals who have one of three characteristics. � First, the individuals may have received more of a drug than is customarily required. � Second, they may have received a conventional amount of the drug, but they may metabolize or excrete the drug unusually slowly, leading to drug levels that are too high. � Third, they may have normal drug levels, but for some reason are overly sensitive to them.

�Type B reactions: tend to be uncommon, not related to dose, unpredictable, and potentially more serious. �They usually require cessation of the drug. They may be due to what are known as hypersensitivity reactions or immunologic reactions. �Alternatively, Type B reactions may be some other idiosyncratic reaction to the drug, either due to some inherited susceptibility (e. g. , glucose 6 -phosphate dehydrogenase deficiency) or due to some other mechanism. �Regardless, Type B reactions are the more difficult to predict or even detect, and represent the major focus of many pharmacoepidemiology

Origin and Evolution of Pharmacoepidemiology �Pharmacotherapy 20 th century �Use of drug inc-----ADR inc � 1961 Maternal use of Thalidomide with malformations (Limb reduction) in offspring -----Focus of detection , prevention and management of ADR----- It began the era of Pharmacoepidemiology �To Identify ADR----spontaneous reporting and surveillance programs created e. g Grey baby syndrome with chloramphenicol �Birth defect with Isotretetinoin

�Drug re-introduce : when drug have unique benefit and risk can be managed �E. g Isotretinoin, cancer drugs etc. �Drug utilization : Define as marketing , distribution , prescription and use of drug in a society with special emphasis on the resulting medical, social and economic consequences �Drug utilization review ( DUR): define as authorized , structural and continuing program that review, analysis and interprets pattern of drug use against predetermined standards �DUR studies focus on drugs and Aim of these studies is to evaluate the appropriateness of therapy using approved criteria and to develop

Aim of Pharmacoepidemiology �Signal Generation: Most commonly associated with ADR but also use to detect new applications �E. g Minoxidil 1 st indicated for hypertension but case report (signal generation) soon identified it causes hirsutism in a number of patients, side effect was investigated and now it is marketed for purpose mainly stimulation of hair growth �Risk Quantification: of ADR often require large sample size

Hypothesis testing: �Require the use of comparison group to determine whethere are difference in variable of interest (risk factor, trait, characteristic, drug exposed, or clinical conditions) �Statistic method are used to assess whether the observed difference could have occur by chance alone �Conclusions about relation b/w exposure to a drug and clinical event thus based on the ability to reject the null hypothesis, postulating that

Reasons to perform pharmacoepidemiology studies


Application of Pharmacoepidemiology �Estimation of risk of drug use �Use in patient counseling �Formulation of public health policy decision �Formulation of therapeutic guidelines and discovery of new indications �Facilitation of pharmaco-economic evaluation



Pharmacoepidemiology in Practice �The basic idea of pharmacoepidemiology is to measure the source, diffusion, use, and effects of drugs in a population and to determine the frequency and distribution of drug use outcomes in that population

The focus of this type of research includes �(1) what is being used (an assessment of specific drugs being used in certain situations) �(2) how it is being used (an assessment of the patterns of use, including how much, where and when, and by whom); and �(3) why it is being used (an assessment of the reasons for drug-taking behaviors and the functions that drugs serve in society).

World Health Organization �WHO focuses its pharmacoepidemiological efforts on ensuring the quality, safety, and efficacy of drugs and their use in specific populations and studies are performed to : �(1) Describe current patterns of drug use in specific patient populations �(2) Determine changes in drug use over time �(3) Measure the effects of information, education, promotional activities, media accounts, and price on drug use �(4) Detect inappropriate drug use and associated problems

Research methods used most often by pharmacoepidemiologists �Cross-sectional study: a prevalence survey of health and illness in the population at one point in time �Case-control study, a retrospective analysis comparing subjects with the condition (cases) to those without it (controls) with respect to possible risk or causative factors �Cohort study, an incidence study that follows a population free of health problems over time, examining subsequent development of problems and factors associated with them. �Clinical trials, an experimental approach that tests the value of a new treatment or intervention

Sources of Data on Drug Use �Institutional record systems and databases �drug utilization studies �hospital-based medical audits (inpatient) �System wide databases �institutionally based reviews (outpatient) �health insurance groups and third-party payers �pharmaceutical organizations �commercial vendors of marketing studies and sales data

�National databases �government-sponsored studies �essential drug lists and inventory data �pharmacoepidemiological surveillance systems �Field data �records of drug dispensers, sellers, and distributors �drug-taking behaviors of individuals and small groups �Experimental data

Problem Solving with Pharmacoepidemiology Medical drug use �Beneficial effects of drug therapy �Risks (e. g. , adverse reactions, side effects) of drug therapy �Inappropriate prescribing behaviours �Patient noncompliance �Irrational self-medication practices �Poor drug use outcomes �Cost-effectiveness of drug therapy

Nonmedical drug use �Social-recreational drug use and associated problems �Acute incidents of drug toxicities (e. g. , overdoses) �Chemical dependencies �Outbreaks and sources of drug epidemics
 Scope of pharmacoepidemiology
Scope of pharmacoepidemiology Pharmacoepidemiology
Pharmacoepidemiology Ad hoc data sources pharmacoepidemiology
Ad hoc data sources pharmacoepidemiology Product scope vs project scope
Product scope vs project scope Io2br3
Io2br3 Example of scope and delimitation
Example of scope and delimitation Scope of psychology
Scope of psychology Define the term pharmacognosy
Define the term pharmacognosy Nature and scope of medical social work
Nature and scope of medical social work Scope of clinical pharmacy in india
Scope of clinical pharmacy in india Jelaskan pengertian ilmu
Jelaskan pengertian ilmu Psycholinguistics definition and scope
Psycholinguistics definition and scope Difference between applied research and basic research
Difference between applied research and basic research Scope of business activities meaning
Scope of business activities meaning Eal reporting resource
Eal reporting resource Orton gillingham workbooks
Orton gillingham workbooks Purpose and scope example
Purpose and scope example Scope of physics
Scope of physics Importance of bioinformatics
Importance of bioinformatics Project charter and scope statement
Project charter and scope statement Executive summary of project charter
Executive summary of project charter What is pharmacognosy definition
What is pharmacognosy definition Explain scope of financial management
Explain scope of financial management Megawords scope and sequence
Megawords scope and sequence