Part I Fetal Circulation ASD VSD Ventricular Septal










- Slides: 18

Part I Fetal Circulation, ASD, VSD

Ventricular Septal Defect • Henri Roger was the first man to describe a ventricular septal defect, in 1879 he wrote: “A developmental defect of the heart occurs from which cyanosis does not ensue in spite of the fact that a communication exists between the cavities of the two ventricles and in spite of the fact that the admixture of venous blood and arterial blood occurs. This congenital defect, which is even compatible with long life, is a simple one. It comprises a defect in the interventricular septum”

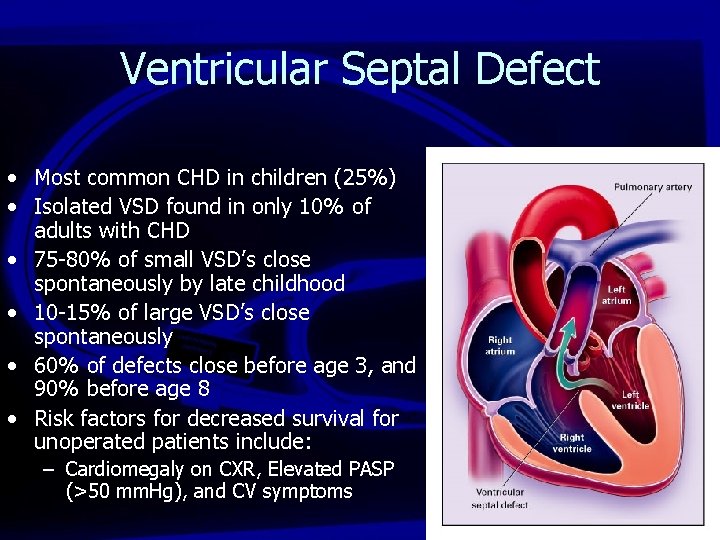
Ventricular Septal Defect • Most common CHD in children (25%) • Isolated VSD found in only 10% of adults with CHD • 75 -80% of small VSD’s close spontaneously by late childhood • 10 -15% of large VSD’s close spontaneously • 60% of defects close before age 3, and 90% before age 8 • Risk factors for decreased survival for unoperated patients include: – Cardiomegaly on CXR, Elevated PASP (>50 mm. Hg), and CV symptoms

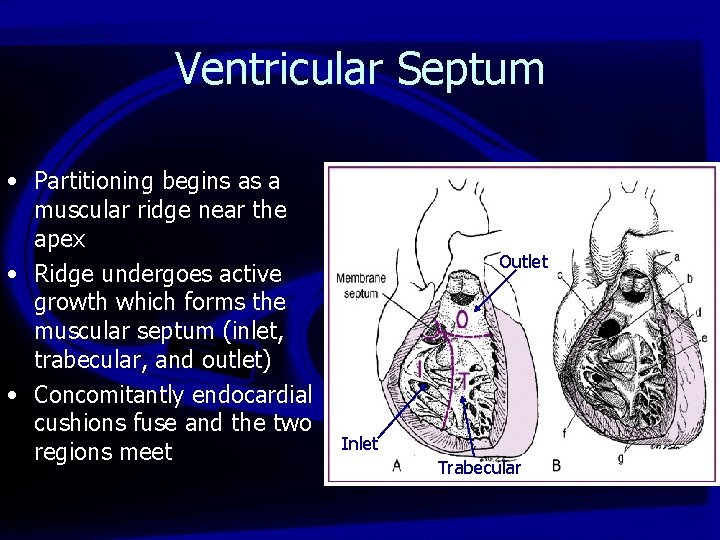
Ventricular Septum • Partitioning begins as a muscular ridge near the apex • Ridge undergoes active growth which forms the muscular septum (inlet, trabecular, and outlet) • Concomitantly endocardial cushions fuse and the two regions meet Outlet Inlet Trabecular

Types of VSD’s • Perimembranous defect (70 -80%) – Less likely to be associated with other defects – Highest rate of spontaneous closure • Muscular or apical defects (5 -20%) – Typically occur in isolation – High spontaneous closure rates unless multiple • AV-Canal type (5 -8%) – Rarely close spontaneously, commonly seen in Trisomy 21 – Usually large & associated with abnormal AV valve • Supracristal or subaortic defects (5 -7%) – Often small but need closure due to associated AR

VSD • Arterial pulse is often normal • There may be a systolic thrill on palpation of the precordium (maximal in 3 rd or 4 th ICS) • Holosystolic, high frequency murmur (grade 46/6) with small VSD and normal PAP • Once PAP increases above the systemic pressures the holosystolic murmur disappears • Increase flow across pulmonary valve causes a SEM • A loud P 2 component is heard in this setting

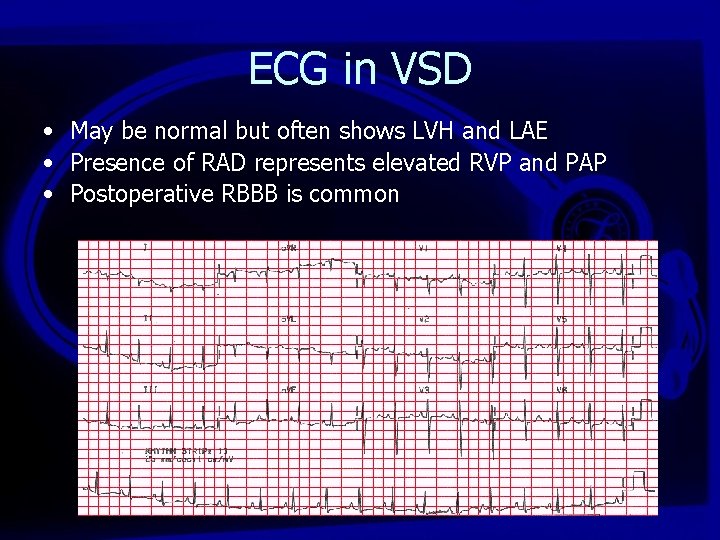
ECG in VSD • May be normal but often shows LVH and LAE • Presence of RAD represents elevated RVP and PAP • Postoperative RBBB is common

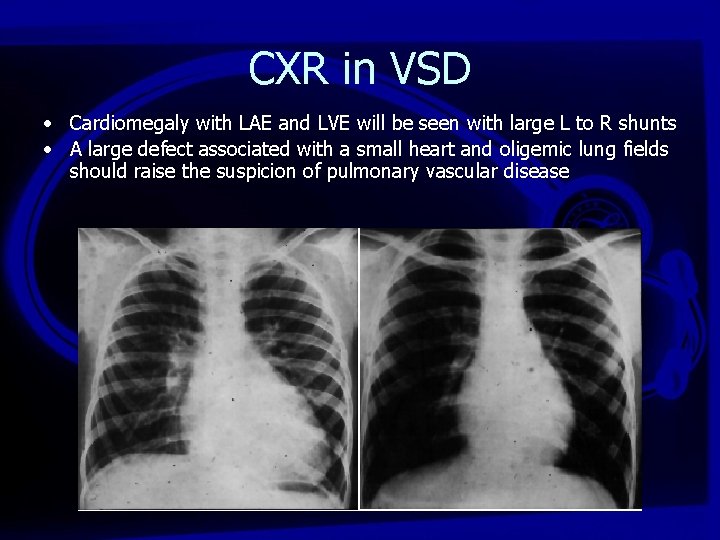
CXR in VSD • Cardiomegaly with LAE and LVE will be seen with large L to R shunts • A large defect associated with a small heart and oligemic lung fields should raise the suspicion of pulmonary vascular disease

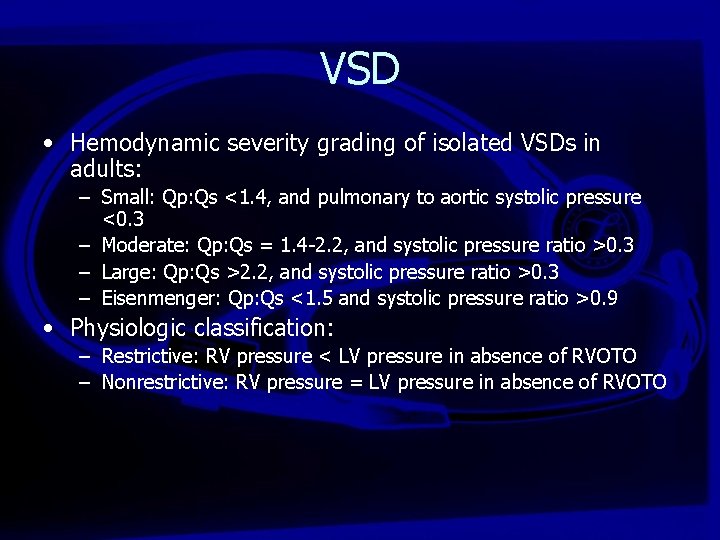
VSD • Hemodynamic severity grading of isolated VSDs in adults: – Small: Qp: Qs <1. 4, and pulmonary to aortic systolic pressure <0. 3 – Moderate: Qp: Qs = 1. 4 -2. 2, and systolic pressure ratio >0. 3 – Large: Qp: Qs >2. 2, and systolic pressure ratio >0. 3 – Eisenmenger: Qp: Qs <1. 5 and systolic pressure ratio >0. 9 • Physiologic classification: – Restrictive: RV pressure < LV pressure in absence of RVOTO – Nonrestrictive: RV pressure = LV pressure in absence of RVOTO

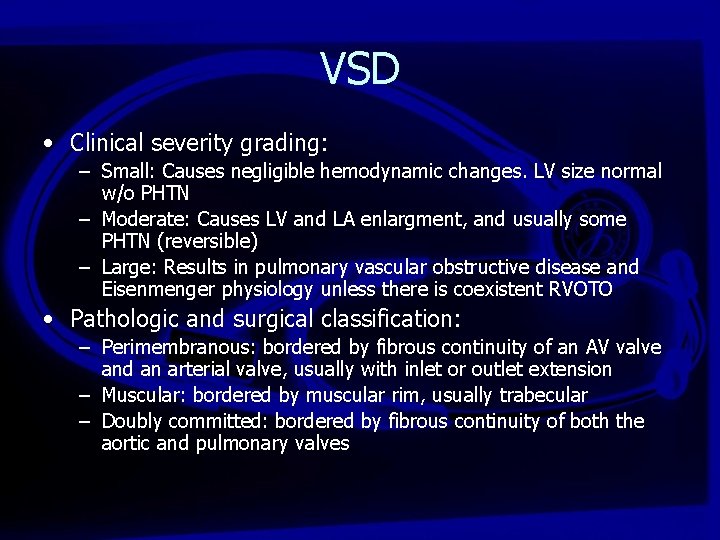
VSD • Clinical severity grading: – Small: Causes negligible hemodynamic changes. LV size normal w/o PHTN – Moderate: Causes LV and LA enlargment, and usually some PHTN (reversible) – Large: Results in pulmonary vascular obstructive disease and Eisenmenger physiology unless there is coexistent RVOTO • Pathologic and surgical classification: – Perimembranous: bordered by fibrous continuity of an AV valve and an arterial valve, usually with inlet or outlet extension – Muscular: bordered by muscular rim, usually trabecular – Doubly committed: bordered by fibrous continuity of both the aortic and pulmonary valves

VSD Repair • When repair is performed in the first two years of life, asymptomatic adult survival with normal growth and development can be anticipated • When surgery is undertaken in older children, a late postopeartive increase in LV chamber size, together with decreased systolic function is seen • Development of late postoperative PHTN is largely determined by the age at surgery and preoperative PVR • Risk of SBE persists and requires prophylaxis

• The initial workup at a minimum should include: – A through clinical assessment – ECG – CXR – TTE/Doppler evaluation

• The diagnostic workup may require: – Oxymetry – Right heart Cath (PAP and PVR determination, to assess pulmonary vascular reactivity) – Coronary angio (in high risk pts or in pts >40 y if surgical repair is planned) – MRI to prove existence of VSD or to assess for other anomalie if doubt remains after other imaging modalities. Also can calculate Qp: Qs – Oxygen saturation with exercise if there is any suggestion of PHTN. Do not exercise if there is severe PHTN or resting oxygen Sat is <85% – Open lung Bx should be considered when the reversibility of PHTN is uncertain from hemodynamic data

• Indications for intervention: Geade C, Level IV – Presence of a significant VSD (symptomatic QP/QS = 2/1, PASP > 50 mm. Hg), deteriorating ventricular fx due to volume (LV) or pressure (RV) overload – Significant RVOTO (pk to pk gradient of > 50 mm. Hg, or instantaneous gradient >70 mm. Hg) – Perimembranous or doubly committed VSD with more than mild AR – In presence of severe PHTN (PAP >2. 3 SABP, or PVR >2/3 SVR), there must be a net L to R shunt of >1. 5 or evidence of PA reactivity when challenged with pulmonary vasodilator, or lung Bx evidence of PA changes are potentially reversible (Heath Edwards grade II-III or less) – Hx of endocarditis especially if recurrent

• Transvenous pacing should be avoided where possible in all patients with VSDs because paradoxical emboli may occur. • Patients with isolated VSD with or without associated lesions RVOTO, AV prolapse, subaortic stenosis, or infective endocarditis) should be repaired by congenital heart surgeons. • Device closure of VSDs may be performed in the setting of isolated trabecular muscular VSDs but are still considered experimental for perimembranous VSDs


• Successful closure is associated with excellent survival if ventricular fx is normal. Elevated PAP preop may progress, regress, or remain the same postop • A. fib may occur, especially if there has been longstanding volume overload of the left heart. Late VT and sudden death are potential risks, especially in patients repaired late in life. CHB may also occur after surgical repair • Pregnancy is well tolerated in women with small or moderate VSD and in women with repaired VSD • Pregnancy is contraindicated in women with Eisenmenger syndrome due to both high maternal (>50%) and fetal (~60%) mortality

• Follow-up: – Patients with following problems benefit from periodic evaluation by cardiologist • Patch leaks or residual VSDs (which seldom require reoperation) • Elevated PVR at time of surgery • Aortic valve surgery • Late repair of moderate or large defects • Significant atrial or ventricular arrhythmias • Associated cardiac lesions (eg RVOTO, AR) – Endocarditis prophylaxis is recommended for 6/12 following VSD closure or for life if residual defect persists