PARACETAMOL OVERDOSE WHATS NEW Tom Heaps June 2019


















- Slides: 32

PARACETAMOL OVERDOSE — WHAT’S NEW? Tom Heaps June 2019

Introduction 2012 CHM (MHRA) Guidance Staggered Overdose Therapeutic Excess(RSTI) Adverse Drug Reactions (ADRs) to NAC Modified NAC Regimen (SNAP) OUTLINE

41, 898 emergency admissions 2017 -2018 (HES data) 167, 822 Hospital TOXBASE page accesses (NPIS Annual Report 2017 -2018) 416 NPIS Consultant referrals (NPIS Annual Report 2017 -2018) 216 deaths in 2017(ONS data) Changes to management guidelines from MHRA 2012 Updated TOXBASE guidance 2017 PARACETAMOL OD

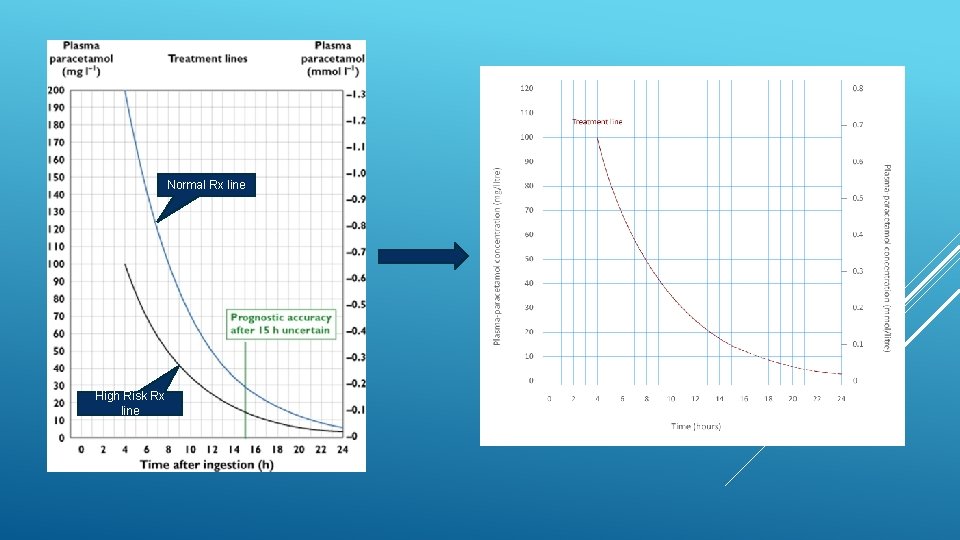
2012 review of NAC in the management of paracetamol overdose Case in 2009 of patient presenting after single overdose of 7 g 4 h paracetamol level 103 mg/L (just above high-risk Rx line) Patient not considered to fall into ‘high-risk’ group ► discharged Represented in fulminant liver failure and died awaiting transplant 9 further UK patients identified since 1991 who died after being initially assessed as not requiring NAC CHM (MHRA) GUIDANCE

1. Use of a single 100 mg L-1 nomogram treatment line 2. No assessment of risk factors to determine need for Rx Assessment of risk factors inconsistent and poor Many risk factors imprecise and difficult to determine with certainty CHM GUIDANCE - CHANGES

Normal Rx line High Risk Rx line

1. Use of a single 100 mg L-1 nomogram treatment line 2. No assessment of risk factors to determine need for Rx 3. Change duration of initial NAC infusion from 15 to 60 mins 4. Treat ALL patients with staggered OD or unknown time of ingestion CHM GUIDANCE - CHANGES

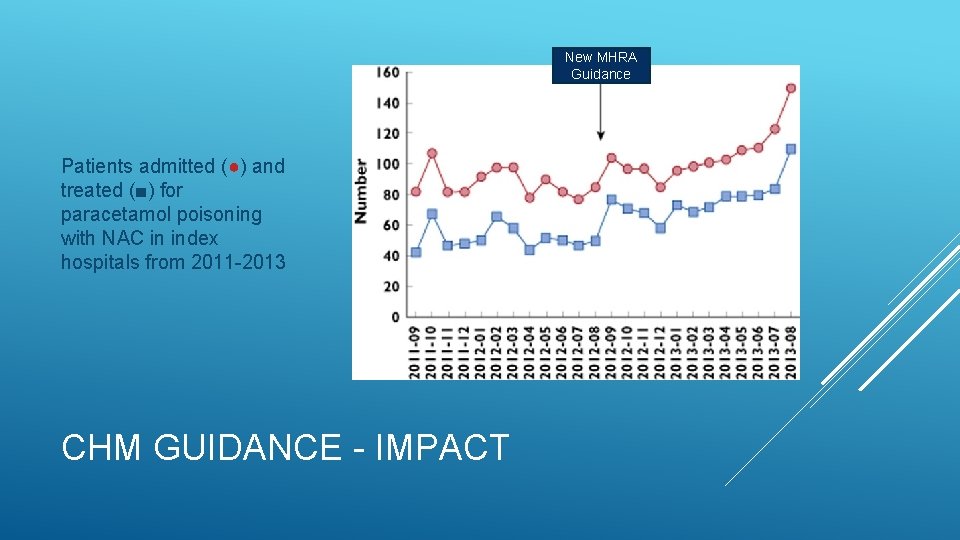
Audit data from 4 UK toxicology units September 2011 – September 2013 Increase in proportion admitted for paracetamol OD (62% → 69%) Increase in proportion treated with NAC (37% → 50%) Bateman DN, Carroll R, Pettie J, et al. Effect of the UK’s revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment. British Journal of Clinical Pharmacology. 2014; 78(3): 610 -618. CHM GUIDANCE - IMPACT

New MHRA Guidance Patients admitted (●) and treated (■) for paracetamol poisoning with NAC in index hospitals from 2011 -2013 CHM GUIDANCE - IMPACT

Audit data from 4 UK toxicology units September 2011 – September 2013 Increase in proportion admitted for paracetamol OD (62% → 69%) Increase in proportion treated with NAC (37% → 50%) Increase in number of patients experiencing ADRs to NAC (323 → 514) No reduction in use of anti-emetic therapy or odds of anaphylactoid reaction Increase in annual cost of managing paracetamol OD (£ 40 m → £ 48. 3 m) Cost per life saved estimated at £ 17. 4 m! Bateman DN, Carroll R, Pettie J, et al. Effect of the UK’s revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment. British Journal of Clinical Pharmacology. 2014; 78(3): 610 -618. CHM GUIDANCE - IMPACT

34 -year-old male (72 kg) with Hx of recurrent DSH 10 x paracetamol (5 g) @ 00: 30 6 x co-codamol 30/500 (3 g) @ 05: 00 1 L of vodka Vomited x 1 Suicidal intent Presents to ED @ 06: 20 Management? CASE 1

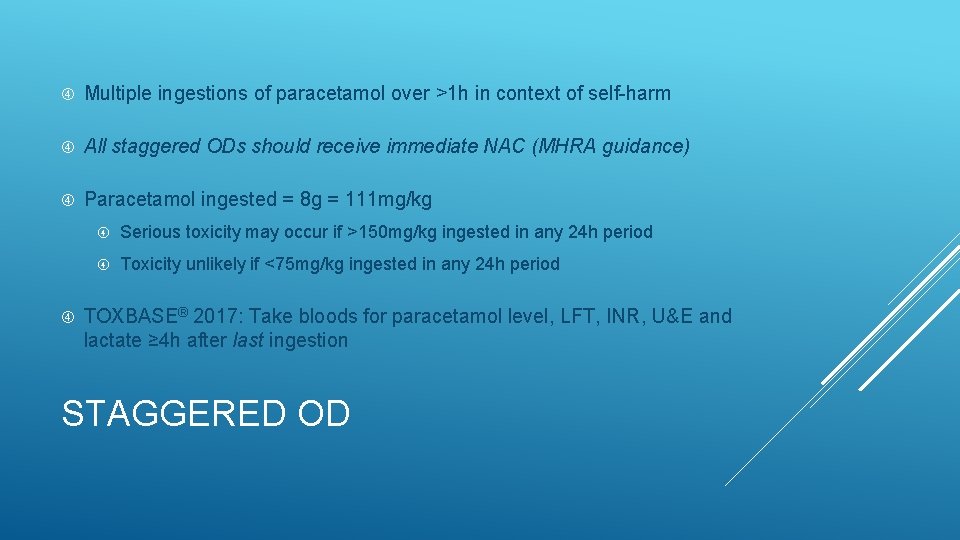
Multiple ingestions of paracetamol over >1 h in context of self-harm All staggered ODs should receive immediate NAC (MHRA guidance) Paracetamol ingested = 8 g = 111 mg/kg Serious toxicity may occur if >150 mg/kg ingested in any 24 h period Toxicity unlikely if <75 mg/kg ingested in any 24 h period TOXBASE® 2017: Take bloods for paracetamol level, LFT, INR, U&E and lactate ≥ 4 h after last ingestion STAGGERED OD

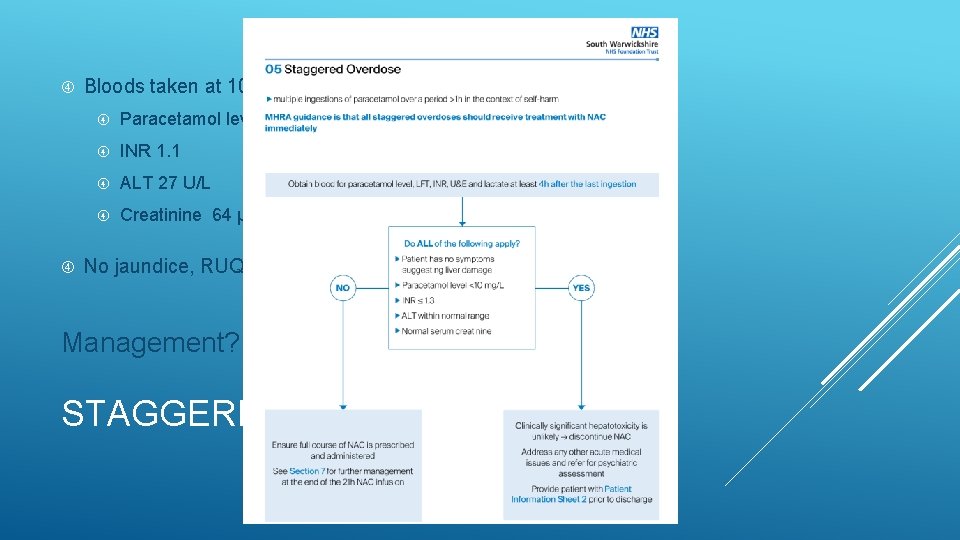
Bloods taken at 10: 00 (5 h after last ingestion): Paracetamol level <10 mg/L INR 1. 1 ALT 27 U/L Creatinine 64 μmol/L No jaundice, RUQ tenderness or encephalopathy Management? STAGGERED OD

41 -year-old female (85 kg) 4 days post-extraction of wisdom teeth Taking paracetamol 1 g every 4 h for last 72 h for pain relief Reduced oral intake secondary to pain Denies suicidal intent Management? CASE 2

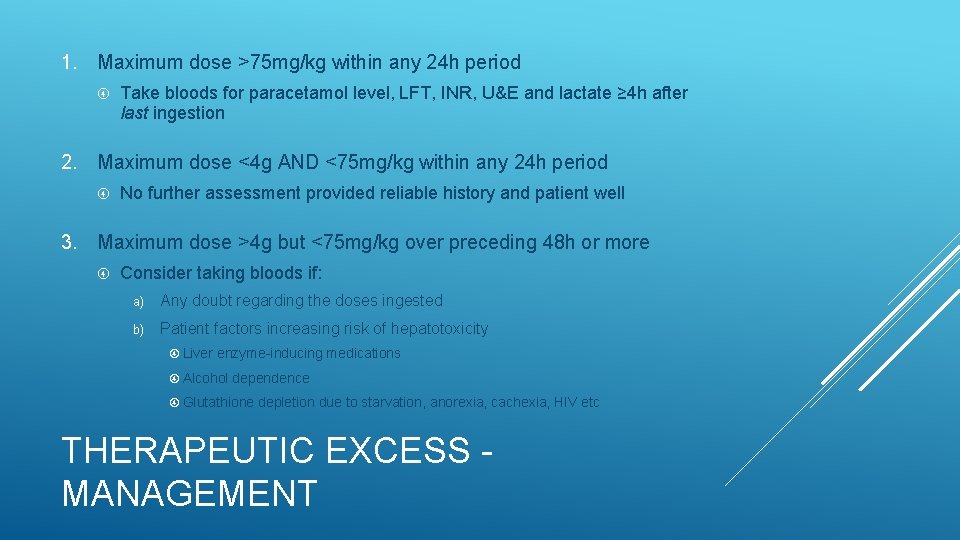
Ingestion of >4 g per 24 h for pain / fever without self-harm intent Treat as staggered OD if any uncertainty regarding intent Paracetamol ingested = 6 g/24 h = 71 mg/kg/24 h Serious toxicity possible if >150 mg/kg ingested in any 24 h period Doses consistently <75 mg/kg in any 24 h period are very unlikely to be toxic but risk may be increased if: This dose is repeatedly ingested over ≥ 48 h Patient factors increasing risk of hepatotoxicity THERAPEUTIC EXCESS(RSTI)

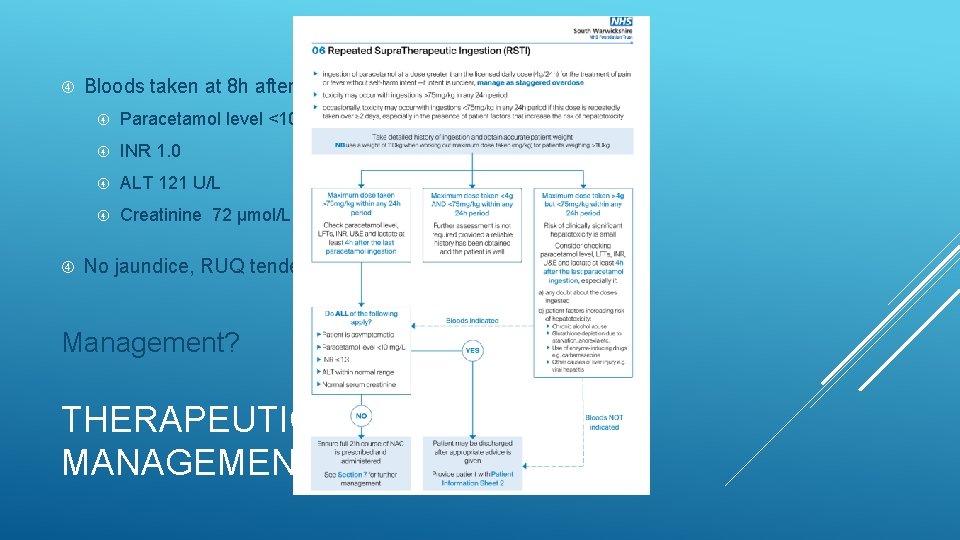
1. Maximum dose >75 mg/kg within any 24 h period Take bloods for paracetamol level, LFT, INR, U&E and lactate ≥ 4 h after last ingestion 2. Maximum dose <4 g AND <75 mg/kg within any 24 h period No further assessment provided reliable history and patient well 3. Maximum dose >4 g but <75 mg/kg over preceding 48 h or more Consider taking bloods if: a) Any doubt regarding the doses ingested b) Patient factors increasing risk of hepatotoxicity Liver enzyme-inducing medications Alcohol dependence Glutathione depletion due to starvation, anorexia, cachexia, HIV etc THERAPEUTIC EXCESS MANAGEMENT

Bloods taken at 8 h after last ingestion: Paracetamol level <10 mg/L INR 1. 0 ALT 121 U/L Creatinine 72 μmol/L No jaundice, RUQ tenderness or encephalopathy Management? THERAPEUTIC EXCESS MANAGEMENT

21 -year-old female (53 kg) Asthmatic Staggered OD 8 g paracetamol (151 mg/kg) Paracetamol level 21 mg/L 10 h after last ingestion 20 minutes into 1 st NAC infusion: Flushing, pruritus, urticaria Vomiting wheeze CASE 3

Occur in up to 30% of patients Nausea, vomiting +/- features similar to anaphylaxis (anaphylactoid) NOT Ig. E-mediated Previous exposure to allergen not required Very rare case reports of fatalities Require additional treatment Extend duration of hospitalisation Anxiety and delays in NAC therapy ADVERSE DRUG REACTIONS (ADRs) TO NAC

Dose-dependent Reactions commonly occur during first bag Inverse correlation with plasma paracetamol concentrations 5 x increased risk if paracetamol levels <100 mg/L Females Atopy or family history of allergy ADRs TO NAC – RISK FACTORS

Temporary cessation of NAC infusion Often all that is required IV chlorphenamine 10 mg +/- nebulised salbutamol No role for systemic corticosteroids Restart infusion as soon as reaction settled Consider slowing rate e. g. giving 1 st bag over 2 h rather than 1 h ADRs TO NAC - MANAGEMENT

Previous reactions to NAC are NOT a contraindication to IV NAC Consider giving 1 st bag at a slower rate e. g. over 2 h Pre-treatment with IV chlorphenamine 10 mg +/- ranitidine 50 mg Nebulised salbutamol if history of bronchospasm ADRs TO NAC – SUBSEQUENT INFUSIONS

Interference with some chemical analysers Underestimation of paracetamol levels by up to 40% Check with lab if measurement during NAC infusion critical Prolongation of INR (PT) up to 1. 3 NAC reduces activity of vitamin K-dependent clotting factors ALT will remain normal OTHER EFFECTS OF NAC

SNAP Scottish and Newcastle Antiemetic Pre-treatment for paracetamol poisoning study

Aimed to determine if adverse effects from NAC could be reduced by: A shorter (12 h), simpler (2 bag) NAC regimen or; Antiemetic (ondansetron) pre-treatment or; Both of the above combined Double-blind randomised study 222 patients across three UK hospitals in 2010 -2012 SNAP

Standard NAC regimen (300 mg/kg over 21 h) Bag 1 (150 mg/kg) over 1 h Bag 2 (50 mg/kg) over 4 h Bag 3 (100 mg/kg) over 16 h Bloods taken at 20 h Modified SNAP regimen (300 mg/kg over 12 h) Bag 1 (100 mg/kg) over 2 h Bag 2 (200 mg/kg) over 10 h Bloods taken at 10 h and extra bag (200 mg/kg) over 10 h if: INR >1. 3 ALT doubled or >100 U/L Paracetamol level >20 mg/L SNAP PROTOCOL

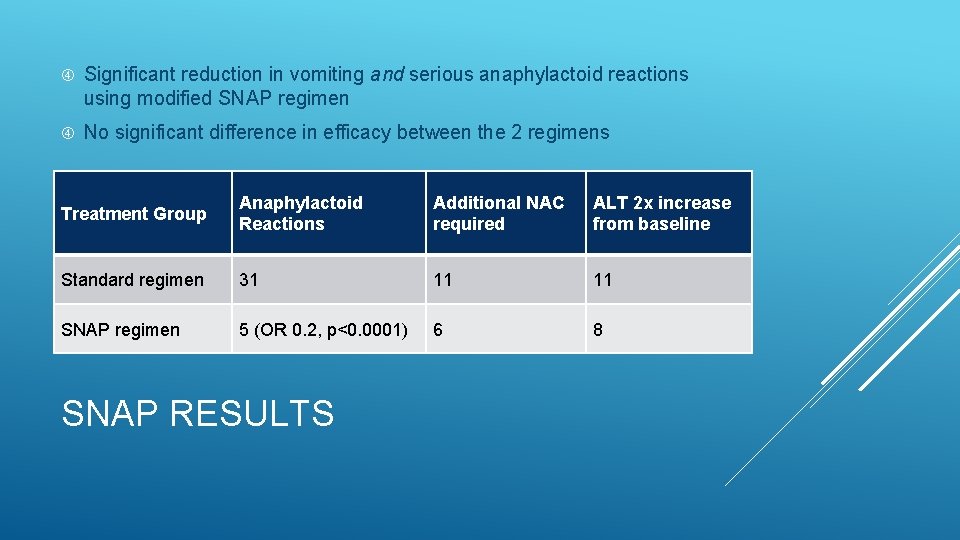
Significant reduction in vomiting and serious anaphylactoid reactions using modified SNAP regimen No significant difference in efficacy between the 2 regimens Treatment Group Anaphylactoid Reactions Additional NAC required ALT 2 x increase from baseline Standard regimen 31 11 11 SNAP regimen 5 (OR 0. 2, p<0. 0001) 6 8 SNAP RESULTS

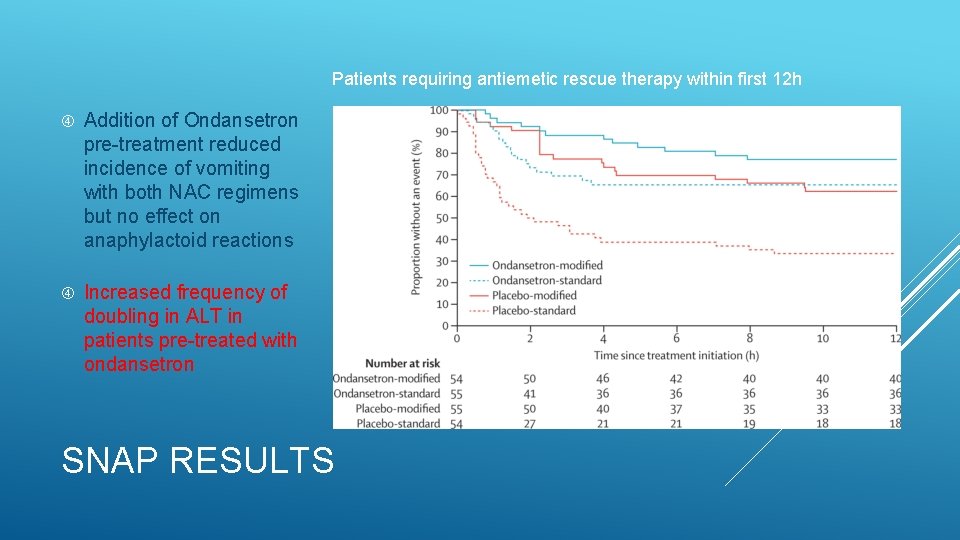
Patients requiring antiemetic rescue therapy within first 12 h Addition of Ondansetron pre-treatment reduced incidence of vomiting with both NAC regimens but no effect on anaphylactoid reactions Increased frequency of doubling in ALT in patients pre-treated with ondansetron SNAP RESULTS

SNAP regimen adopted into routine practice by RIE (2015), STH and RVI (2016) Treat with 12 h SNAP Regimen ≥ 24 h after last paracetamol ingestion ALT >2 x ULN or; ALT doubled from admission or; INR >1. 3 AND ALT >ULN or; Paracetamol level >20 NO Take bloods at 10 h <24 h after last paracetamol ingestion or timing uncertain Take bloods at ≥ 24 h after last ingestion or time of admission ALT >2 x ULN or; ALT doubled from admission or; INR >1. 3 AND ALT >ULN or; Paracetamol level >20 NO Administer further NAC at 200 mg/kg over 10 h YES Take bloods Medically fit for discharge NO ALT >2 x ULN or; ALT doubled from admission or; INR >1. 3 AND ALT >ULN or; Paracetamol level >20 YES

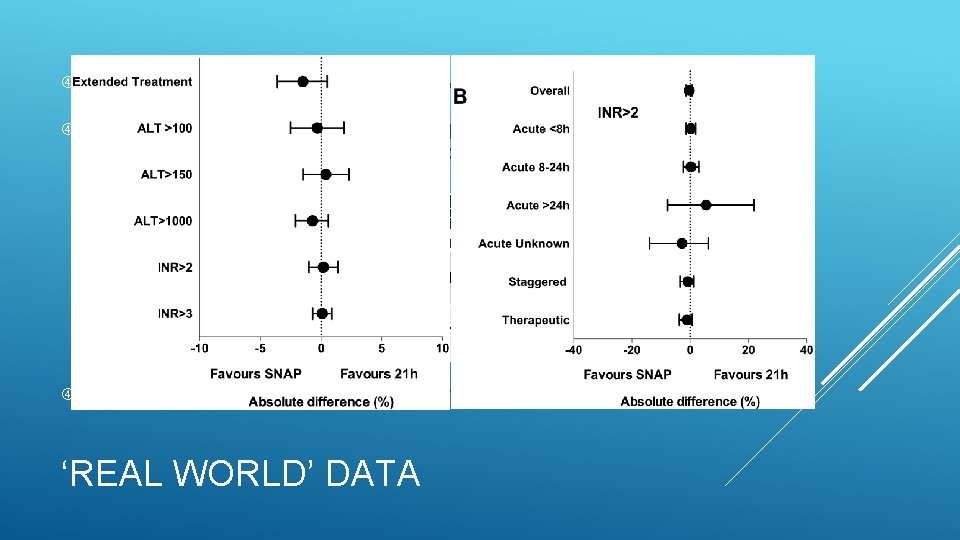
Data from 1852 patients treated with SNAP regimen Compared with data collected from 1488 patients treated with conventional 21 h NAC regimen for 2 y prior to SNAP Treatment Group Anaphylactoid Reaction Extended Treatment >21 h Hepatotoxicity (ALT >1000) Standard regimen n=1488 163 (11%) 160 (11%) 64 (4. 3%) SNAP regimen n=1852 37 (2%) 171 (9%) 67 (3. 6%) No deaths or readmissions with liver failure within 30 d in either group ‘REAL WORLD’ DATA

Increased safety (NNT 10 to prevent one anaphylactoid reaction) Similar efficacy to standard NAC regimen Simpler regimen (less potential for medication errors) Reduction in length of hospital stay and costs More individualized therapy – massive overdoses will receive 480 mg/kg in 21 h with SNAP vs 300 mg/kg with traditional regimen Further research required to define criteria for immediate discharge after completing 12 h SNAP regimen Currently not licenced by MHRA but watch this space… SNAP BENEFITS

QUESTIONS ?
 Acute liver failure criteria
Acute liver failure criteria Tom heaps
Tom heaps June 21 2019 geometry regents
June 21 2019 geometry regents 2019 june calendar
2019 june calendar Vitamin b6 overdose symptoms
Vitamin b6 overdose symptoms Tricyclic antidepressants overdose
Tricyclic antidepressants overdose Magnesium overdose
Magnesium overdose Dan muse
Dan muse Normal range calcium
Normal range calcium Zoplicone overdose
Zoplicone overdose Opioid overdose
Opioid overdose Opioid overdose
Opioid overdose Opioid overdose
Opioid overdose Cholinomimetic
Cholinomimetic Dcjs virginia gov
Dcjs virginia gov Function of vitamin a
Function of vitamin a Soft drink stand
Soft drink stand Go 910
Go 910 The devil and tom walker symbols
The devil and tom walker symbols Irpwm 2020
Irpwm 2020 Love heaps
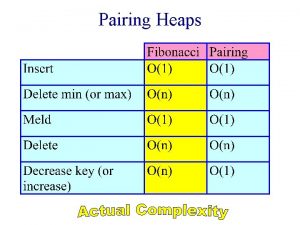
Love heaps Lazy binomial heaps
Lazy binomial heaps Skew heap
Skew heap Stratus cumulus nimbus
Stratus cumulus nimbus Fibonacci
Fibonacci Reheap up and reheap down
Reheap up and reheap down Soft heaps of kaplan and zwick uses
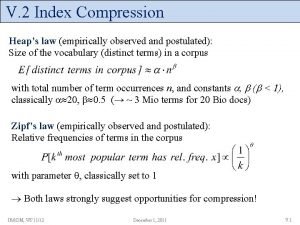
Soft heaps of kaplan and zwick uses Heap's law
Heap's law Febs letters
Febs letters Mecanismo de accion del paracetamol
Mecanismo de accion del paracetamol Sifat fisika dan kimia paracetamol
Sifat fisika dan kimia paracetamol Uniporte ejemplo
Uniporte ejemplo Paracetamol adverse effects
Paracetamol adverse effects