Nuclear Chemistry I Nuclear Radiation the nuclei of

- Slides: 6

Nuclear Chemistry I. Nuclear Radiation -the ______ nuclei of some ______ atoms are _______ unstable and undergo _______ nuclear ____ reactions 1895 _______ German _____ physicist ____ Wilhelm -in _____, Roentgen discovered that _____ invisible ____ rays that he called ______ X-rays were emitted from the surface of certain materials when bombarded with ____ electrons they were _____ Wilhelm Roentgen (1845 -1923) An X-ray taken by Roentgen http: //en. wikipedia. org/wiki/Wilhelm_R%C 3%B 6 ntgen

Nuclear Chemistry I. Nuclear Radiation -in _____, 1896 _______ French _____ physicist _____ Henri _____ Becquerel discovered that uranium _______ salts produced ______ spontaneous ____ emissions that ____ darkened ______ photographic ______ plates Henri Becquerel (1852 -1908) -______ Marie _____ Curie named the process that _____ Becquerel observed ______, radioactivity and in _____, 1898 _____ Marie and Pierre _____ Curie identified two new ____, elements ______ Radium and Polonium first _____ radioactive ________, the _________ elements to be identified -_____ Henri _____, Becquerel ______ Pierre _____, Curie and ______ Marie _____ Curie shared the ______ 1903 ______ Nobel _____ Prize for Physics and in _____, 1911 ______ Marie _____ Curie became the _______, first person to win ____ two ______ Nobel ______ Prizes when ____ Prize for _____ Chemistry she won the Nobel _____ http: //en. wikipedia. org/wiki/Henri_Becquerel

Nuclear Chemistry II. Radioactive Decay -the ______ electrostatic repulsion ____ caused by protons _______ in the _______ nucleus is overcome by the ______ strong _______ nuclear _____, force which is created by the interaction of _____ called subatomic particles quarks which make up _______ protons and neutrons ______, _______, and ______, gluons which ______ “glue” _______ quarks together Nobel Prize winners Marie and Pierre Curie in 1904 with future Nobel Prize winner Irène (Joliot. Curie) strong ______ nuclear _____ force is _______ released during the -the _______ nuclear ____ reactions that ______ power the ____ Sun and _____, stars _______ nuclear ______ power ______ plants and ______ nuclear ______ bombs http: //www. aip. org/history/curie/recdis 2. htm

Nuclear Chemistry II. Radioactive Decay -_______ nuclear ____ stability depends on the _______ balance between ______ electrostatic ____ repulsion and ______ strong _______ nuclear _____ force Mushroom cloud over the city of Nagasaki, Japan, on August 9, 1945, due to the explosion of the atomic bomb “Fatman”. 42, 000 people died instantly, and 40, 000 more were injured -______ electrostatic _____ repulsion _____ increases with an increase in _______ number of _______, protons but ____ electrostatic _____ repulsion does not _______ increase with ______ an increase _______ in _______ number of ____, neutrons while ______ strong _______ nuclear _____ force does, so the _______neutron ___-_______ to proton _____ ratio in _____ atoms with < 20 protons must be around ____ 1: 1 in order for the _______ nucleus to be _______, stable while the neutron to _______-_____ proton _____ ratio must be around _____ 1. 5: 1 in order for the _______ with nucleus to be _______ stable in atoms ____ < 20 _______ protons

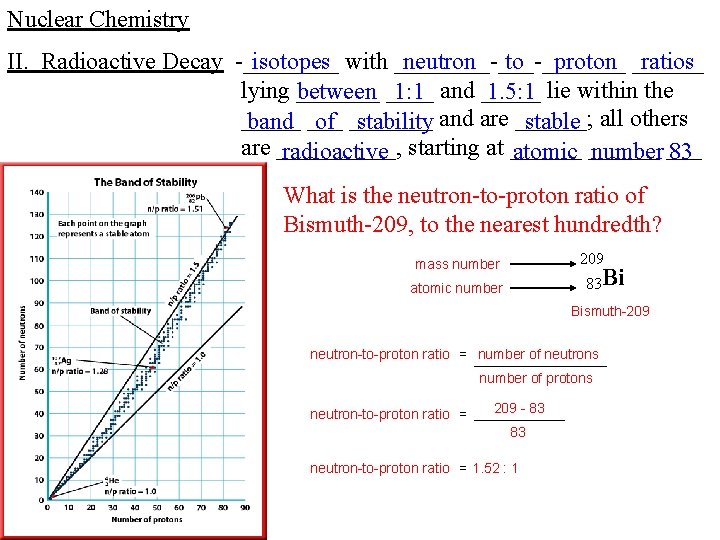
Nuclear Chemistry II. Radioactive Decay -____ isotopes with ____-_______ neutron to proton ______ ratios lying _______ between ____ 1: 1 and _____ 1. 5: 1 lie within the _____ band ___ of _______ stability and are ______; stable all others are _____, radioactive starting at ______ atomic ______ number ___ 83 What is the neutron-to-proton ratio of Bismuth-209, to the nearest hundredth? 209 mass number 83 Bi atomic number Bismuth-209 neutron-to-proton ratio = number of neutrons number of protons neutron-to-proton ratio = 209 - 83 83 neutron-to-proton ratio = 1. 52 : 1

http: //pro-resources. net/images/mapofplants. gif http: //en. wikipedia. org/wiki/Three_Mile_Island_accident