NonFerrous Metals and their alloys Nonferrous metals are










- Slides: 12

Non-Ferrous Metals and their alloys. Non-ferrous metals are those which do not contain iron. In general they have excellent resistance to corrosion. Copper, reputed to be the first metal used by mankind, includes some very useful and interesting properties, it is malleable and ductile. It is for this reason is was of such interest to our ancestors. Another non-ferrous metal, aluminium, has only featured in engineering terms in the last 75 years. (C) Coláiste Lorcáin Engineering 1

Copper as an engineering metal came to prominence during the industrial revolution, up to then it was considered only for its decorative value and utensils value and on roofing. Properties of copper include • Malleability - the ability to be beaten or rolled into shape. • Ductility - The ability to be drawn into shape as in the manufacture of copper wire. • Copper is a good conductor of heat and electricity. (C) Coláiste Lorcáin Engineering 2

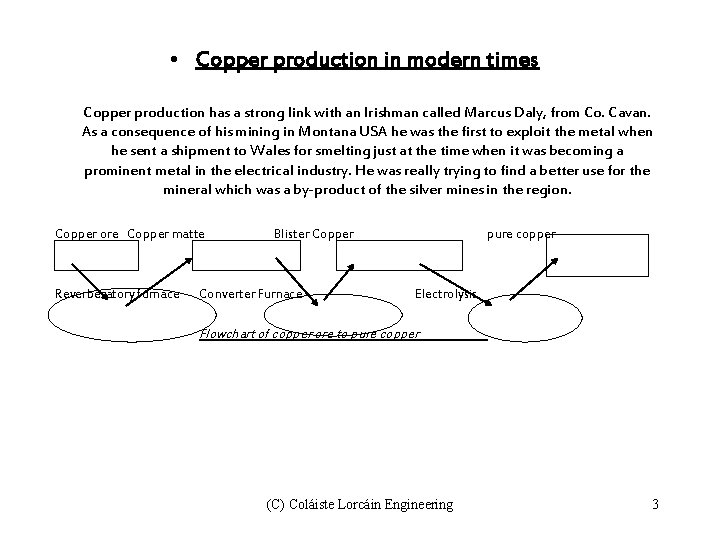
• Copper production in modern times Copper production has a strong link with an Irishman called Marcus Daly, from Co. Cavan. As a consequence of his mining in Montana USA he was the first to exploit the metal when he sent a shipment to Wales for smelting just at the time when it was becoming a prominent metal in the electrical industry. He was really trying to find a better use for the mineral which was a by-product of the silver mines in the region. Copper ore Copper matte Reverberatory furnace Blister Copper Converter Furnace pure copper Electrolysis Flowchart of copper ore to pure copper (C) Coláiste Lorcáin Engineering 3

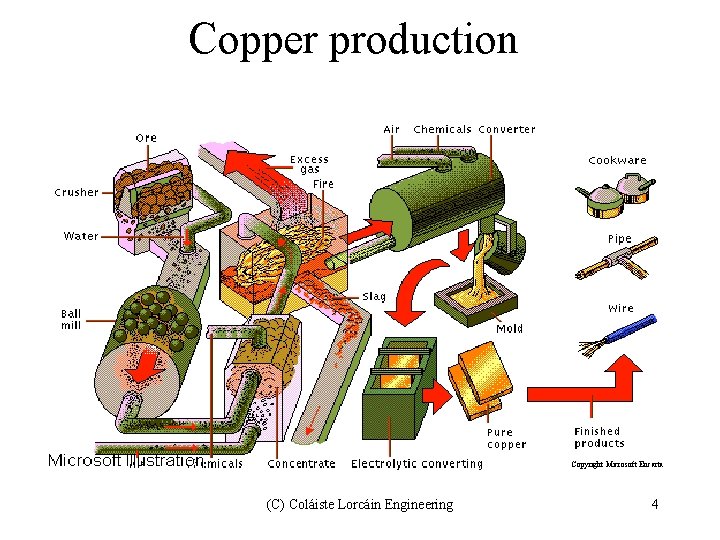
Copper production Copyright Microsoft Encarta (C) Coláiste Lorcáin Engineering 4

The production of copper. There are three distinct stages to the production of copper; 1 Copper ‘Matte’ - The first stage of the production of copper is with matte. It is a mixture of 30% to 40% copper. Ore is first concentrated at the mining site by crushing and then leaching or floatation. The ore is then roasted or smelted in an oil fired furnace at a temperature below the melting point of copper, [1083 o. C] The resultant matte contains high levels of sulphur and iron from the chalcopyrite (Cu Fe S 2) 2 Converting - Iron and sulphur are removed by blowing air through the molten matte in a converter vessel, similar to the Bessemer converter. The matte is placed in the converter and held at the temperature for about 8 hours. Blowing continues until only copper sulphide remains. This is what is referred to as ‘Blister Copper’. It is up to 99% pure. This is then refined by further heating to remove the oxygen. It is then cast into ingots known as ‘Anodes’. 3. Refining by Electrolysis: - For copper to be used in the electrical industry it has to have 99. 99% purity. This can only be achieved by electrolytic refinement. Pto. (C) Coláiste Lorcáin Engineering 5

Refined blister copper anodes are attached to the positive DC supply, while a thin cathode plate is connected to the negative supply. An electrolyte, a mixture of copper sulphate and sulphuric acid at 50 O C is poored into the tanks. Acurrent density of around 200 amps is passed between the anode and the cathode, depositing pure copper onto the thin cathodes Application of copper Over half of all copper produced is used in the electrical industry. Copper wire for transmission accounts for a large amount of this use. Central heating and plumbing are the major applications of copper. Cylinders, copper piping etc are the most widely used. Recycling of copper is an industry in itself and accounts for 40% of all copper used. (C) Coláiste Lorcáin Engineering 6

Aluminium is one of the most versatile of metals. It is only of engineering significance since the late twenties, when it became possible to produce it in commercial quantities. Next to steel it is the most used metal in the world. Yet the production of aluminium is only 6% that of steel. Aluminium has a wide range of uses, from cooking foil to aircraft. Its properties make it suitable for many applications. It is light does not corrode, is a good conductor of electricity and heat and is cheaper than copper. Aluminium can be cast, extruded, rolled, forged, drawn, etc. , to give us the numerous shapes of the aluminium objects that we see everyday. (C) Coláiste Lorcáin Engineering 7

Mining Aluminium is the most abundant metal on the earth’s crust. About 8% of the earth’s crust is made up of aluminium. So why is it not the most abundant metal in use? There is great difficulty and cost attached to refining aluminium. It does not exist as a pure metal in nature, but is combined with other elements. The Ore of aluminium is called ‘Bauxite’, [hydrated aluminium oxide]. Bauxite is mined in many countries, France, Australia, Guinea, Brazil, Russia and china being the more common. Mining Methods; Open-cast mining is carried out using very large scale plant. After the overburden of topsoil is removed the ore is excavated out and loaded into giant dumper trucks. The ore is crushed and washed close to the mining site to save on transport costs. Production of Aluminium from Barxite; It requires a huge amount of energy to extract aluminium from its ore. The energy is supplied in the form of electricity. Because of the large amount of electricity involved the production of aluminium is generally in countries with large amounts of cheap electricity, such as Canada, Norway and Brasil. (C) Coláiste Lorcáin Engineering 8

A Two-stage process. Purification; Before aluminium can be made the ore has to be purified. After being mixed with caustic soda solution the bauxite is sent to heated pressure vessels where the alumina hydrated aluminium oxide dissolves in the caustic soda. The impurities are removed as red mud. As the alumina cools alumina hydrate forms crystals. It is then roasted or calcined. In the Bayer process. This process is what is carried out in Aughinis in the Shannon Estuary outside Limerick. Reduction of Alumina to Aluminium; Electrolysis is used to convert alumina to aluminium. Alumina has a melting point of 2000 o. C. To reduce the temperature at which conversion takes place cryolite is added and hence reduces the amount of energy required to convert the alumina to aluminium. It takes about 2 tonnes of alumina , 15000 units of electricity to make one tonne of aluminium. (C) Coláiste Lorcáin Engineering 9

Recycling of Aluminium Recycling accounts for about 25% of the total world production of aluminium. As the amount of bauxite is limited, it makes good sense to recycle the metal. The cost of recycling a tonne of aluminium is 5. 3% the cost of its initial production. Aluminium and its uses; Aluminium is often used in transmission cables for high tension systems in place of copper because of cost factors. It is also better in national grid transmission because of the reduction of weight. Aluminium is often alloyed with other metals such as copper, magnesium, nickel and zinc to produce metals with special properties. (C) Coláiste Lorcáin Engineering 10

Lead is one of those metals which have been used for many ages. The Romans used lead to duct water to their baths. Batteries are the main users of lead nowadays as it has found to be dangerous in many other applications. Petrol used lead to aid the lubrication of the fuel system. Lead is a toxic material and is being replaced by other materials in places like drainpipes, guttering etc. Lead-tin alloys of solder are essential to the electrical industry and are known as softsolders(approx 60%tin-40% lead). Other uses of lead include the sheathing of underground telecommunication cables, bearing matals, radiation protection shields (X-ray and Nuclear). (C) Coláiste Lorcáin Engineering 11

Tin is mainly used in the production of tinplate( sheet steel coated in tin for corrosion protection. Tin was mined mainly ion Britain in Cornwall which attracted the Romans to Britain. Tinplate is produced by dipping sheet steel into baths of molten tin. The canned or tinned food industry is based on the tinplate can. The tin makes up just 1% of the tincan. Zinc is a white silvery metal which is a poor conductor of both electricity and heat. It is mostly used as a source of protecting steel I the form of galvanised steel. It is usually applied by hotdipping the steel in vats of molten zinc, But is also applied by electroplating. Zinc is used in dry-cell batteries such as those for torches and radios etc. It is alloyed with other metals to make die castings. One of its main uses is its alloy with copper to make brass. (C) Coláiste Lorcáin Engineering 12
 Insidan region jh
Insidan region jh Metal categories
Metal categories Metals on periodic table
Metals on periodic table Metal vs nonmetal
Metal vs nonmetal Combining materials grade 5
Combining materials grade 5 Natural science grade 7 matter and materials
Natural science grade 7 matter and materials Non metal example
Non metal example Application and processing of metal alloys
Application and processing of metal alloys Applications and processing of metal alloys
Applications and processing of metal alloys Aluminum and its alloys
Aluminum and its alloys Aluminum and its alloys
Aluminum and its alloys Moscow institute of steel and alloys
Moscow institute of steel and alloys Ferrous material
Ferrous material